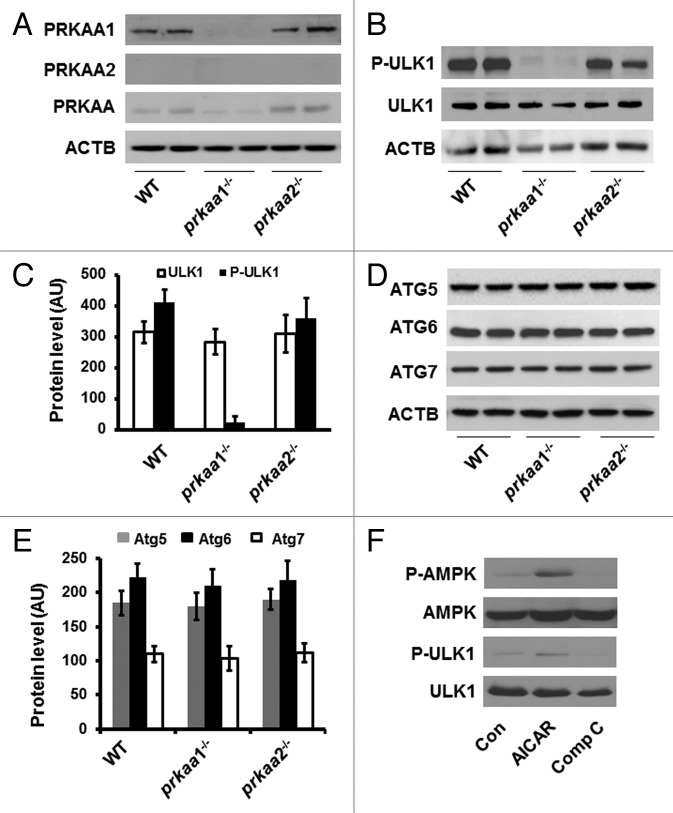
Figure 1. Deletion of prkaa1 inhibits ULK1 phosphorylation. (A) Cell lysates prepared from WT, prkaa1−/−, and prkaa2−/− TFRC+ and GYPA+ erythroid precursors were subjected to western blot analyses to detect the expression of PRKAA1, PRKAA2, and total PRKAA. (B) Expression of total and phosphorylated ULK1 (Ser555) in mouse TFRC+ and GYPA+ erythroid precursors was analyzed by western blotting. (C) The expression of ULK1 was quantified by densitometry and normalized to ACTB (n = 4). (D and E) ATG protein levels in mouse TFRC+ and GYPA+ erythroid precursors were detected by western blotting (D) and quantified by densitometry (E). (F) K562 cells were treated with AICAR (2 mM) or Compound C (10 µM) for 4 h, and the cell lysates were subjected to western blotting to determine the phosphorylation of PRKAA (Thr172) and ULK1 (Ser555). The blot shown is representative of 3 independent replicates. Con, control; Comp C, compound C.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.