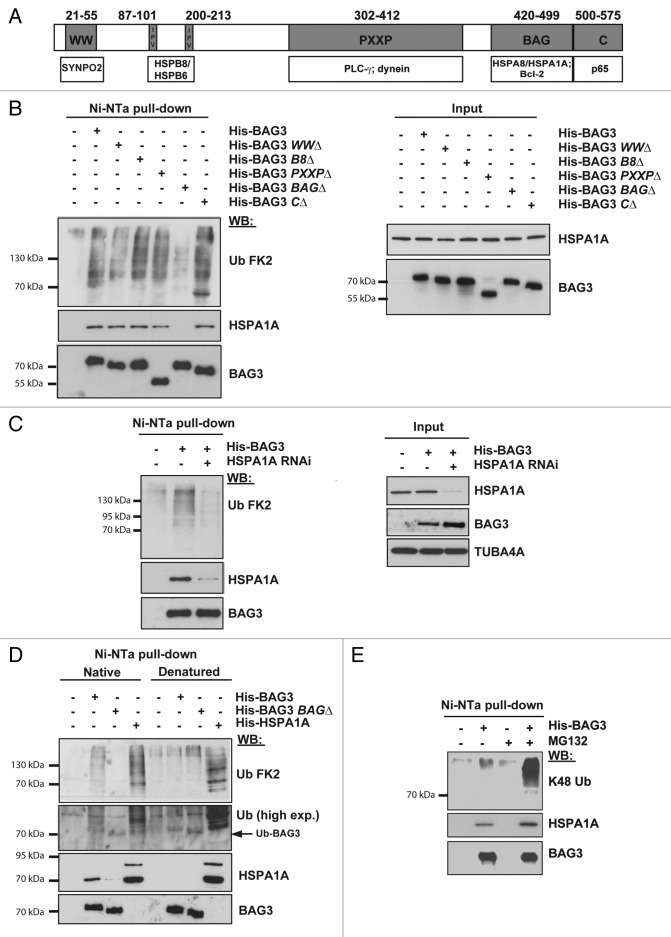
Figure 2. BAG3 binds to (poly)ubiquitinated proteins in an HSPA8- and HSPA1A-dependent manner. (A) Schematic representation of the binding domains and partners of BAG3. (B) HEK293T cells were transfected with an empty vector or vectors encoding His-tagged FL BAG3 or deletion mutants each lacking one specific binding domain (WWΔ, B8Δ, PxxPΔ, BAGΔ, or CΔ). Twenty-four hours post-transfection cells were lysed and subjected to purification of His-tagged BAG3 with Ni-NTA beads. (C) HEK293T cells were transfected with a control siRNA (−) or a siRNA directed against HSPA1A (+). Forty-eight hours post-transfection, cells were transfected with an empty vector or a vector encoding His-tagged BAG3. Cells were subjected to Ni-NTA affinity isolation as described above. (D) HEK293T cells transfected with an empty vector or vectors encoding His-tagged FL BAG3, BAG3-BAGΔ, or HSPA1A were subjected to Ni-NTA affinity isolation under native or denatured conditions. Levels of (poly)ubiquitinated proteins, BAG3 and HSPA1A were analyzed in the affinity isolated fractions. (E) HEK293T cells transfected with an empty vector or vectors encoding His-tagged FL BAG3 were either left untreated or treated with 20 μM MG132 for 3 h and subsequently subjected to Ni-NTA affinity isolation. (B–E) Levels of (poly)ubiquitinated proteins, BAG3, and HSPA1A were analyzed in the affinity isolated and/or input fractions. Concerning ubiquitin, while the FK2 ubiquitin antibody was used in (B–D), an antibody specific for K48 ubiquitin was used in (E).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.