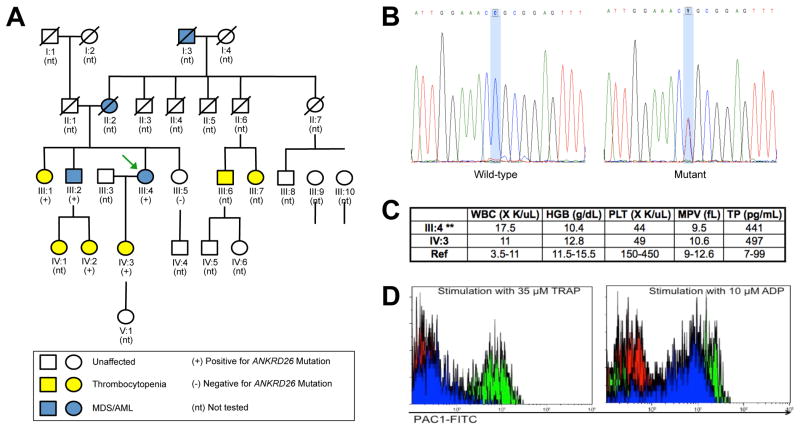
Figure 1.
Characterization of the family and its germline ANKRD26 mutation. (A) Five-generation family pedigree, showing the mother (III:4; green arrow) and daughter (IV:3) pair. Family members with thrombocytopenia are shown in yellow, those with MDS/AML are in blue, and those tested for the presence of the ANKRD26 mutation are indicated by + (mutation present), or − (mutation absent). (B) Electropherograms of the sequenced wild-type control versus the proband with an ANKRD26 c.-118 C>T mutation. (C) Pertinent laboratory results of the mother and daughter are shown: WBC, white blood count; HGB, hemoglobin in g/dL; PLT, platelet count; MPV, mean platelet volume; TP, thrombopoietin in pg/mL; Ref, reference range. **Note that the blood counts obtained from the mother were analyzed when she had MDS. (D) Platelet flow cytometry. Red: control plus PBS; Green: control plus agonist; blue: patient (III:4) plus agonist. Left panel: Assay run with Thrombin Receptor Activation Peptide (TRAP) as the agonist; Right panel: Assay run with ADP as the agonist. Platelet activation was quantified by the binding of FITC-labeled PAC-1 IgM antibody (BD-Biosciences, New Jersey, USA), which selectively recognizes the activated conformation of the platelet fibrinogen receptor, GPIIb/IIIa. This approach interrogates platelets at the receptor activation step, which occurs just prior to actual aggregation in the presence of fibrinogen. Results are expressed as the percentage of events in the platelet gate that exceed the defined positivity threshold for PAC-1 binding.