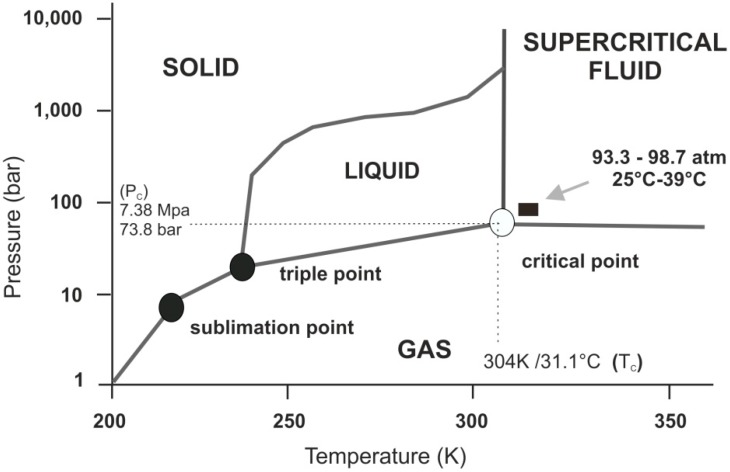
Figure 1.
Schematic p-T phase diagram of CO2. Note if the temperature and pressure of a substance are both higher than Tc and Pc for a particular substance, the substance is defined as a supercritical fluid. Carbon dioxide has four distinct phases; the standard solid, liquid and gas phase as well as the supercritical phase. Carbon dioxide transitions to supercritical phase occur relatively readily at the critical points of 7.38 MPa, 304 K/31.1 °C and 73.8 bar.