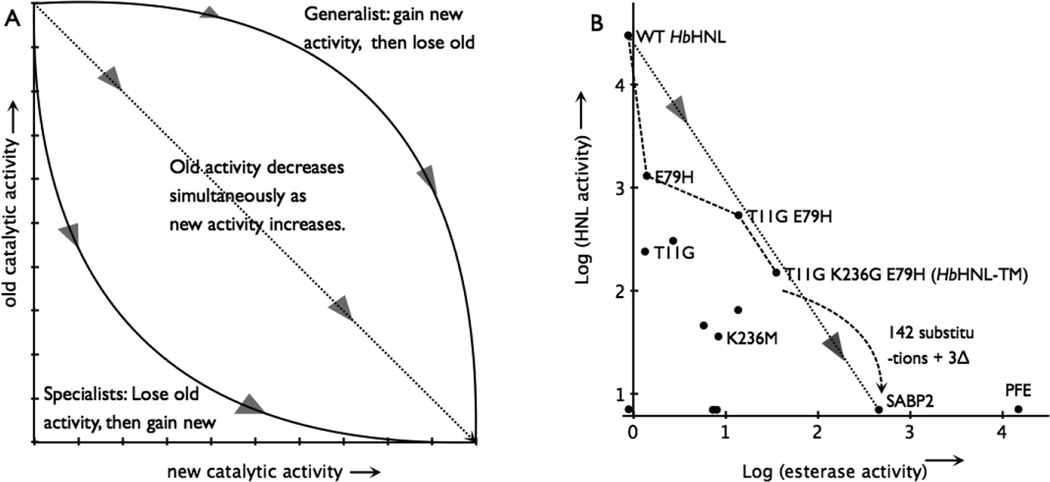
Figure 4.
Switching an enzyme from one activity to another. A) The hypothetical paths to replace the old catalytic activity with the new one. The triangles show the direction of evolution. Adapted from reference 18. B) The best path from a hydroxynitrile lyase (HbHNL) to an esterase (SABP2) lies below the dotted line connecting the activities of the two natural enzymes indicating that esterase activity drops more quickly than hydroxynitrile lyase activity increases. The axes mark the detection limit for each enzymatic activity. The black dots indicate all the variants and natural enzymes tested in this paper, but for clarity only some of them are labelled. Activities are log10 of specific activity in units of mU/mg; 5 mM mandelonitrile and 0.3 mM pNPAc. Adding an additional 142 amino acid substitutions and 3 insertions to HbHNL-TM would create the SABP2 sequence.