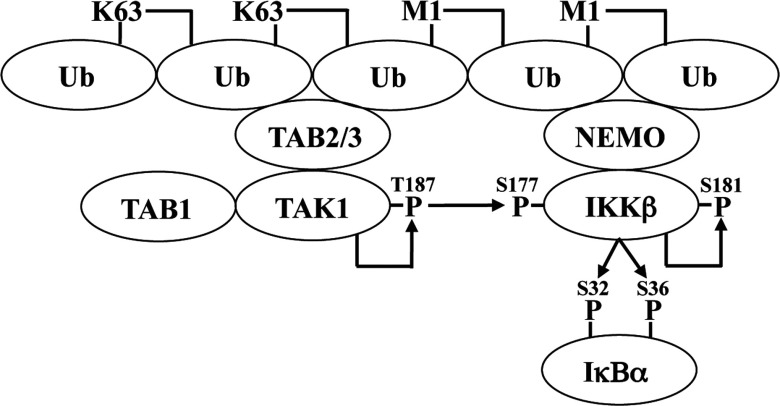
Figure 5. Proposed mechanism for the activation of IKKβ by IL-1 in IKKα-deficient MEFs.
IL-1 stimulates the formation of hybrid ubiquitin chains in which LUBAC-generated Met1-linked ubiquitin oligomers are attached covalently to TRAF6-generated Lys63-linked ubiquitin oligomers. The binding of Lys63-linked ubiquitin to TAB2 or TAB3 activates the TAK1 complex by inducing autophosphorylation of the catalytic subunit at Thr187 [31]. The M1-linked ubiquitin chains interact with NEMO permitting TAK1 to phosphorylate IKKβ at Ser177. Phosphorylation of Ser177 allows autophosphorylation of Ser181. The activated IKKβ can then phosphorylate IκBα. Sites of phosphorylation are denoted by ‘P’.