Fig. (1).
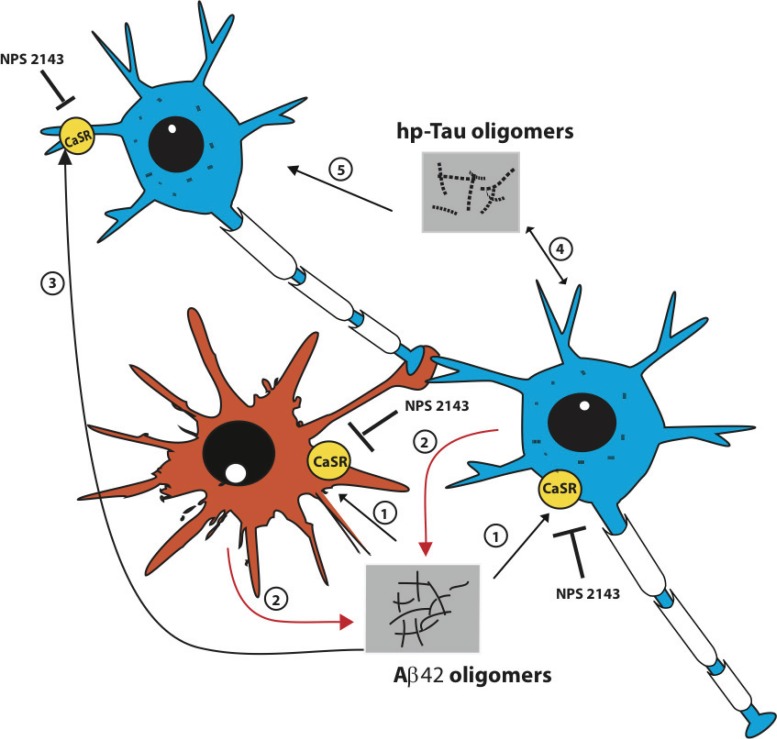
The interactions between exogenous Aβs and the CaSRs located on the plasma membrane of astrocyte-neuron teams advance the extracellular release and spread of Aβ42 and hp-Tau oligomers, the “AD infectious seeds”. Two neurons and one astrocyte of the same team are schematically depicted here. The neuropathology begins when for aging-related causes Aβ42 monomers accumulate in the extracellular space, oligomerize, and bind the CaSRs inserted in the plasma membranes of both cell types (1). The engendered Aβ●CaSR signaling induces the intracellular accrual (not shown) and oversecretion of de novo produced Aβ42 monomers from both neurons and astrocytes (2). The Aβ42 monomers oligomerize, spread, bind, and activate the CaSRs of a further neuron of the team (3). By doing this the Aβ42 oligomers cause the additional release of surplus Aβ42 moieties from this and other neurons (not shown). These vicious cycles can be unceasingly repeated and hence recruit ever-increasing numbers of astrocyte-neuron teams and thus inexorably advance the progression of AD. Hp-Tau oligomers formation is also triggered in the Aβ42-exposed neurons by still unclear mechanisms that might be at least partially driven by pathological Aβ●CaSR signaling (4). Secreted hp-Tau oligomers are next either taken up by the secreting cells or transferred to contiguous neurons (and maybe astrocytes too) to hinder microtubule functions and help destroy synaptic spines (5). Once produced, hp-Tau oligomers are also capable of an independent self-induction and spreading. A highly selective allosteric CaSR antagonist (calcilytic) like NPS 2143 can completely suppress the manifold noxious effects driven by pathological Aβ42●CaSR signaling both in neurons and in astrocytes thereby restoring conditions close if not identical to physiological ones [5,6]. Other relevant effects of the pathological Aβ42●CaSR signaling, like the surplus production and secretion of NO and VEGF-A from the astrocytes, which are also suppressed by calcilytic NPS 2143 [6,38], and the extracellular accrual of Aβ42 oligomers, which activate microglia, damage oligodendrocytes, and cause cerebral amyloid angiopathy, have been omitted from the picture for the sake of clarity.