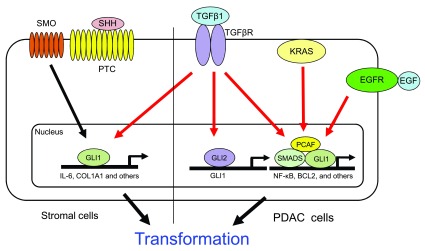
Figure 1. Schematic Representation of Pathways Modulating GLI1-expression/activity in Cancer.
In canonical HH signaling in stromal cells, binding of the SHH ligand to Patched (PTC) activates the Smoothened (SMO) receptor, which induces GLI1 activation of its target genes, including IL-6 and COL1A1 (Martin E Fernandez-Zapico unpublished observation), leading to pre-malignant lesions. SMO-independent mechanisms for regulation of GLI1 in PDAC include KRAS, TGFβ, and EGFR. GLI1 has been shown to regulate the NF-κB pathway in a HH-independent manner downstream of KRAS, leading to pancreatic epithelial transformation. TGFβ promotes GLI2 expression in PDAC through Smad3 and β-catenin/LEF-TCF-dependent upregulation of GLI2 independent of HH signaling. TGFβ induced GLI2 expression, and subsequent GLI1 activation, is associated with EMT, tumor growth, and metastasis. TGFβ can also modulate GLI1 activity by promoting the formation of a transcriptional complex with the TGFβ-regulated transcription factors, SMAD2 and 4, and PCAF, at the BCL2 promoter in cancer cells to regulate TGFβ-induced gene expression. EGFR signaling is aberrantly activated in a majority of PDACs. EGFR and HH have been demonstrated to act synergistically to promote cancer cell initiation and growth by modulation of gene expression of distinct novel pathways through a GLI1-dependent mechanism.