Abstract
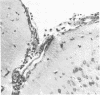
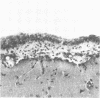
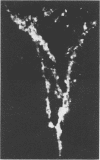
The pathogenesis of experimental vaccinia virus infection in weanling mice after intracerebral inoculation was followed with virological, histological, and immunohistological methods. High-dose inoculation, virus spread from brain to thoracic and abdominal viscera probably by an undetected early viremia. Virus did rise to detectable levels in blood by day 5 and was found to be associated with the mononuclear cell fraction. By day 12, 30% of the animals had died and no further deaths occurred. Rise of neutralizing antibody correlated with disappearance of cell-free virus in blood, brain, and viscera. Virus was present in the brains of animals for 20 days after inoculation. This animal model may be useful to study mechanisms of persistent central nervous system virus disease relevant to man.
Full text
PDF

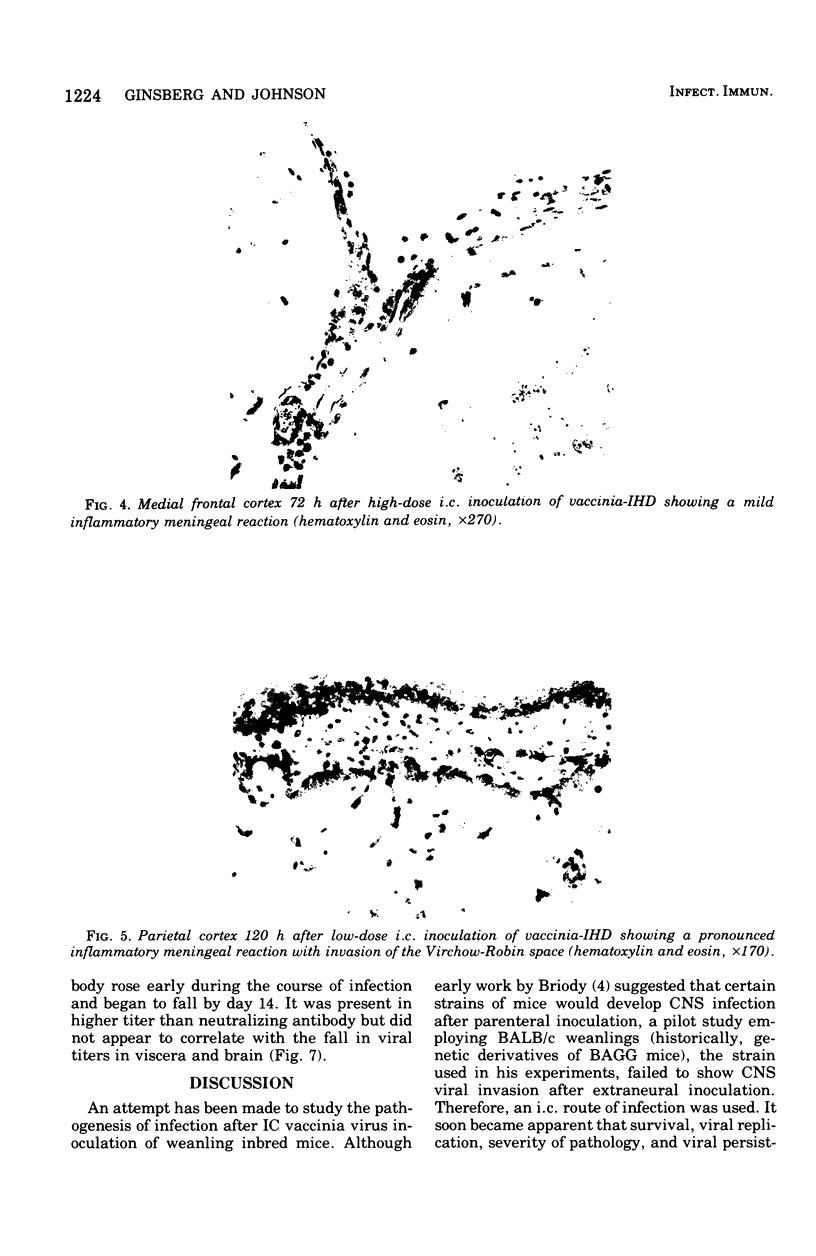
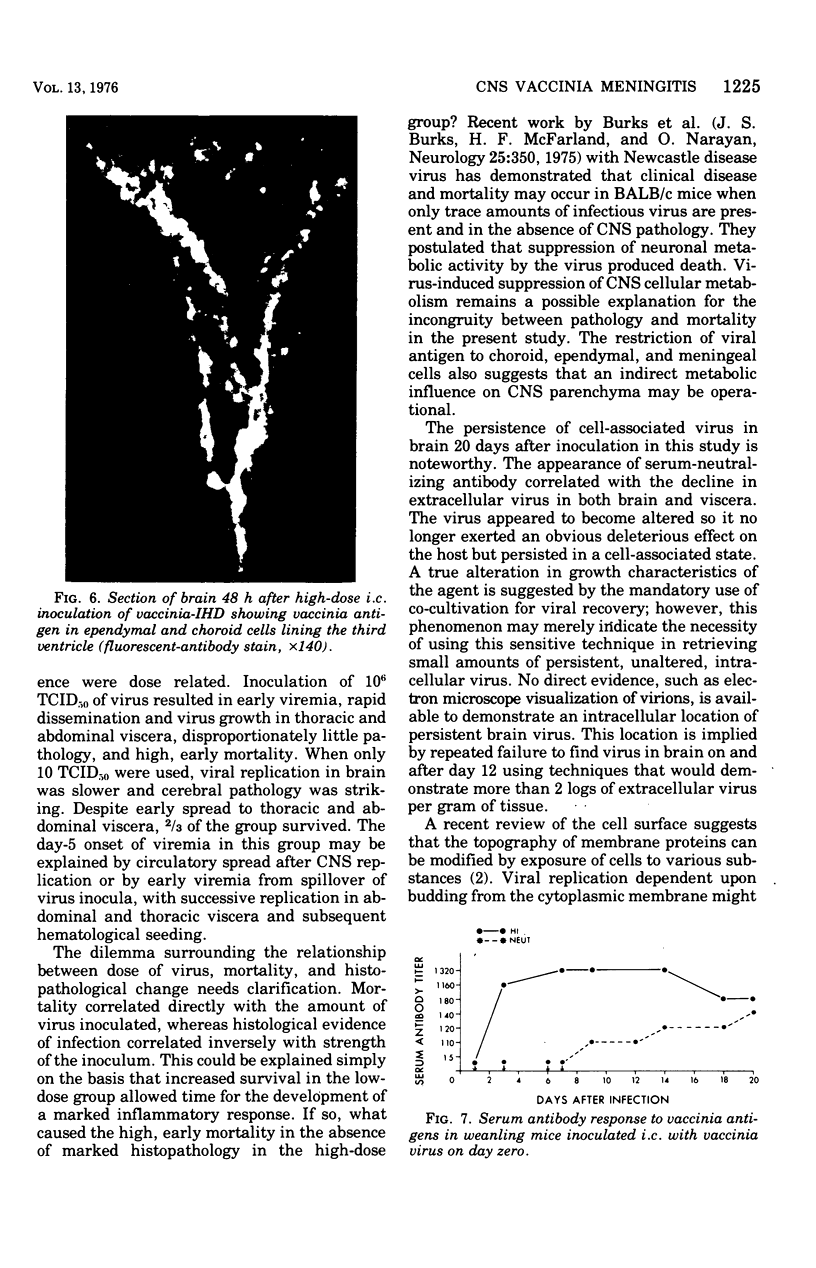


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIODY B. A. Response of mice to ectromelia and vaccinia viruses. Bacteriol Rev. 1959 Jun;23(2):61–95. doi: 10.1128/br.23.2.61-95.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Ukena T. E., Yin H. H. The cell surface. N Engl J Med. 1975 Mar 6;292(10):515–520. doi: 10.1056/NEJM197503062921007. [DOI] [PubMed] [Google Scholar]
- CASSEL W. A. Multiplication of vaccinia virus in the Ehrlich ascites carcinoma. Virology. 1957 Jun;3(3):514–526. doi: 10.1016/0042-6822(57)90007-7. [DOI] [PubMed] [Google Scholar]
- FLICK J. A., PINCUS W. B. Inhibition of the lesions of primary vaccinia and of delayed hypersensitivity through immunological tolerance in rabbits. J Exp Med. 1963 Apr 1;117:633–646. doi: 10.1084/jem.117.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN R. M., BARONS, BUCKLER C. E., STEINMULLER R. I. The role of antibody, delayed hypersensitivity, and interferon production in recovery of guinea pigs from primary infection with vaccinia virus. J Exp Med. 1962 Sep 1;116:347–356. doi: 10.1084/jem.116.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS J. L., MILLER H. G., STANTON J. B. Para-infectious encephalomyelitis and related syndromes; a critical review of the neurological complications of certain specific fevers. Q J Med. 1956 Oct;25(100):427–505. [PubMed] [Google Scholar]
- Gurvich E. B., Movsesyants A. A., Stepenenkova L. P. Isolation of vaccinia virus from children with postvaccinal encephalitis at late intervals after vaccination. Acta Virol. 1975 Jan;19(1):92–92. [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Possible mechanisms in the pathogenesis of "postinfectious" encephalomyelitis. Res Publ Assoc Res Nerv Ment Dis. 1971;49:342–355. [PubMed] [Google Scholar]
- Hirsch M. S., Murphy F. A. Effects of anti-lymphoid sera on viral infections. Lancet. 1968 Jul 6;2(7558):37–40. doi: 10.1016/s0140-6736(68)92904-8. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Nahmias A. J., Murphy F. A., Kramer J. H. Cellular immunity in vaccinia infection of mice. Anti-thymocyte serum effects on primary and secondary responsiveness. J Exp Med. 1968 Jul 1;128(1):121–132. doi: 10.1084/jem.128.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. 3. Induction of defective measles infection in hamster brain. Exp Mol Pathol. 1974 Oct;21(2):166–178. doi: 10.1016/0014-4800(74)90087-2. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Glasgow L. A. Factors modifying host resistance to viral infection. 3. Effect of whole body x-irradiation on experimental encephalomyocarditis virus infection in mice. J Exp Med. 1968 May 1;127(5):1035–1052. doi: 10.1084/jem.127.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Hirsch M. S., Kramer J. H., Murphy F. A. Effect of antithymocyte serum on herpesvirus hominis (type 1) infection in adult mice. Proc Soc Exp Biol Med. 1969 Nov;132(2):696–698. doi: 10.3181/00379727-132-34290. [DOI] [PubMed] [Google Scholar]
- Neff J. M., Lane J. M., Pert J. H., Moore R., Millar J. D., Henderson D. A. Complications of smallpox vaccination. I. National survey in the United States, 1963. N Engl J Med. 1967 Jan 19;276(3):125–132. doi: 10.1056/NEJM196701192760301. [DOI] [PubMed] [Google Scholar]
- Paterson P. Y. Immune processes and infectious factors in central nervous system disease. Annu Rev Med. 1969;20:75–100. doi: 10.1146/annurev.me.20.020169.000451. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Shabo A. L., Petricciani J. C., Kirschstein R. L. Identification of herpes simplex and vaccinia viruses in corneal cell cultures with immunoperoxidase: a light and electron microscopic study. Invest Ophthalmol. 1973 Nov;12(11):839–847. [PubMed] [Google Scholar]
- Soekawa M., Morita C., Moriguchi R., Nakamura M. Neurovirulence of vaccinia viruses for mice. Zentralbl Bakteriol Orig A. 1974 Jun;226(4):434–442. [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Worthington M., Rabson A. S., Baron S. Mechanism of recovery from systemic vaccinia virus infection. I. The effects of cyclophosphamide. J Exp Med. 1972 Aug 1;136(2):277–290. doi: 10.1084/jem.136.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]