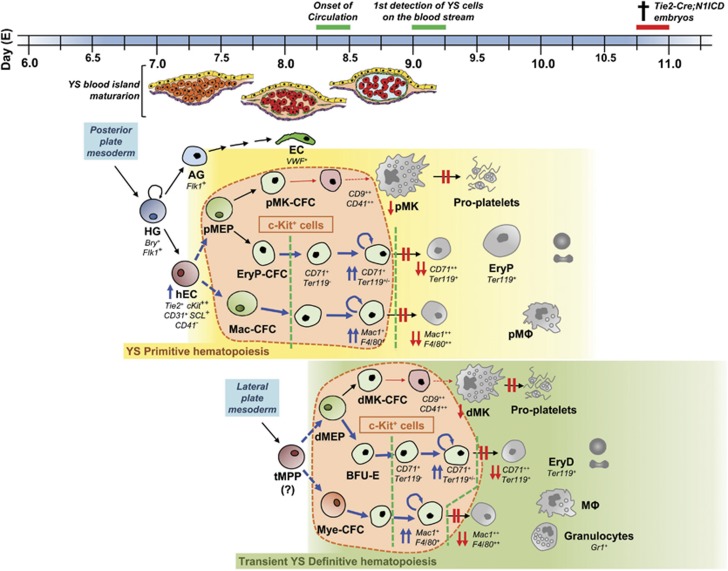
Figure 7.
Yolk sac hematopoiesis alterations in Tie2-Cre;N1ICD embryos. In the mouse at around embryonic day (E) 7.0–7.25 early hemangioblasts (HG) emerged from the posterior streak mesoderm and give rise to hemogenic endothelial cells (hEC), and angioblasts (AG) progenitor cells on the extraembryonic YS blood island. The AG will produce part of the YS vascular endothelial cells (EC). On the other hand, YS hematopoiesis occurs in two waves, the primitive (from ∼E7.25, yellow box) and definitive (green box) waves that overlap, temporally and spatially, and produce relatively short-lived cells. hEC give rise, first, to bipotential primitive megakaryocyte/erythroid progenitor (pMEP) that will in turn generate specific primitive progenitors for both cell lineages (pMK-CFC and EryP-CFC); and second, to primitive macrophage precursor cells (Mac-CFC). These progenitors will generate the primitive hematopoietic cell lineages: primitive erythrocytes (EryP), primitive megakaryocytes (pMK) – able to give rise to proplatelets – and primitive macrophages (pMΦ). This short-lived primitive wave is rapidly followed by the second hematopoietic wave that comprises YS definitive erythroid, megakaryocyte and several myeloid lineages. The YS definitive populations are believed to arise from transient multipotent progenitors (tMPPs) – but not from hematopoietic stem cells, which emerge in the mouse YS at around E9.0 – that are detected from ∼E8.25 and generate different transient cell populations of restricted erythro-myeloid progenitors (EMPs). EMPs comprise definitive-type bipotential megakaryocyte/erythroid progenitor (dMEP) that will give rise to transient definitive megakaryocyte and erythrocyte progenitors (dMK-CFC and BFU-E) and definitive myeloid progenitors (Mye-CFC), from which definitive-type erythrocytes (EryD), megakaryocytes (dMK) – with the capacity to originate proplatelets – and several myeloid lineages: macrophages (MΦ), granulocytes and mast cells will be produced. The expression of N1ICD in early Tie2+ progenitors produces severe alterations in both YS− primitive and definitive hematopoiesis characterized by high frequency of c-Kit-expressing multipotential and lineage-restricted progenitors (encircled by the orange bubbles) at E9.5, whereas it blocks the generation of the last maturation stages of erythrocytes, megakaryocytes and myeloid lineages. First, no CD71++TER119+ and Mac1++F4/80++ cells are produced. In contrast, lineage-restricted progenitors accumulate in the initial or intermediate stages of their differentiation (CD71++TER119− proerythroblasts and Mac1+F4/80+ myeloid progenitors). Accordingly, diminished numbers of CD9++CD41++ megakaryoblasts are found among EGFP+ YS cells and the final steps of differentiation in vitro are selectively blocked. On the other hand, ectopic N1ICD expression in these intermediate progenitors (delimited by green open lines) induces misbalanced proliferation and apoptosis, preventing their normal differentiation. Thick blue arrows denote an increase in cell frequency; weak red arrows indicate a restriction on cell frequency