Abstract
Leukemia therapeutics are aiming for improved efficacy by targeting molecular markers differentially expressed on cancerous cells. Lymphocyte function-associated antigen-1 (LFA-1) expression on various types of leukemia has been well studied. Here, the role and expression of LFA-1 on leukemic cells and the possibility of using this integrin as a target for drug delivery is reviewed. To support this rationale, experimental results were also included where cIBR, a cyclic peptide derived from a binding site of LFA-1, was conjugated to the surface of polymeric nanoparticles and used as a targeting ligand. These studies revealed a correlation of LFA-1 expression level on leukemic cell lines and binding and internalization of cIBR-NPs suggesting a differential binding and internalization of cIBR-NPs to leukemic cells overexpressing LFA-1. Nanoparticles conjugated with a cyclic peptide against an accessible molecular marker of disease hold promise as a selective drug delivery system for leukemia treatment.
Keywords: HL-60 cell line, LFA-1, leukemia, Molt-3 cell line, Molt-4 cell line, peptide, nanoparticles, targeting, U937 cell line
FUNCTION OF LFA-1 AND ITS ROLE IN DISEASES
Leukocyte function associated antigen-1 (LFA-1) is a heterodimeric protein comprised of alpha (Mw = 180 kDa) and beta (Mw = 95 kDa) subunits. It is a member of the integrin family constitutively expressed on all immune cell subsets including leukocytes (1–4). LFA-1 plays a role in many immunological response processes including adhesion and transmigration of leukocytes through endothelium and participates in the immunological synapse between T cells and antigen presenting cells in T cell activation (2, 5). The activation of T cells can induce proliferation and differentiation of T cells (2, 6). The major binding ligand of LFA-1 is intercellular adhesion molecule-1 (ICAM-1)(7). LFA-1 activation is tightly regulated through intracellular signaling (inside-out signaling) and it is a prerequisite for ligand binding (2). The binding of LFA-1 to its ligand triggers intracellular signaling leading to conformational change of LFA-1 from a low affinity to a high affinity form which has a greater accessibility to its ligand and allows tighter binding(2). In addition to the high affinity conformation, the binding of LFA-1 to its ligand may also be enhanced by clustering of LFA-1 (2, 8). The cluster formation is initiated by multivalent binding of LFA-1 to ICAM-1, hence strengthening cell-cell adhesion.
The interaction between LFA-1 and ICAM-1 plays an important role in the regulation of a variety of immune responses(9). LFA-1 is important for leukocyte endothelial adhesion and extravasation to inflammatory sites. It also plays a key role in co-stimulation required for T cell activation. Accordingly, the functions of LFA-1 are relevant to most immunological diseases or symptoms such as inflammation, autoimmune diseases and graft rejection after organ transplantation (1, 10). The diseases are often typified by overexpression or deficiency of LFA-1. Several molecules including antibodies, peptides and small molecules have been developed to block the binding of LFA-1 and ICAM-1 (10). Efalizumab(Raptiva®), a recombinant anti-human LFA-1 antibody was a prescribed medication used as an immunosuppressive agent in psoriasis (11). However, Raptiva® has been associated with an increased risk of progressive leukoencephalopathy(PML) which is a fatal disease of the central nervous system, therefore; it was withdrawn from the market in mid-2009 (12). Both monotherapy of anti-LFA-1 and combination therapy with anti-ICAM-1 antibody were effective in promoting long-term survival of islet, cardiac, small bowel and nerve allografts in animal models (1, 13–17). Treatment with anti-LFA-1 antibodies has shown disease prevention in animal models with autoimmune encephalomyelitis and autoimmune diabetes and has shown benefits in lupus and inflammatory arthritis (1, 18–22).
LEUKEMIA AND TARGETED DRUG DELIVERY
Leukemia is a malignant disease originating in the bone marrow. An abnormal proliferation of blood cells leads to an excess of these cells entering the blood stream (23). Leukemia has a higher mortality rate than other types of cancer among children and young adults under the age of 20 (24). Currently, most forms of leukemia are treated with pharmaceuticals, typically by combination chemotherapy regimens. Patients may also be treated with radiation therapy or bone marrow transplantation, however, these treatments are generally more invasive(25). Drug discovery for treating leukemia is focused on enhancing efficacy, while minimizing therapy-related toxicity. A promising therapeutic strategy is selective targeting of molecular markers of malignant cells, which are not expressed or are expressed to a lesser extent on normal human hematopoietic stem cells or lymphocytes (25).
Progress in therapeutic interventions leading to improved disease outcomes is primarily a result of the continued development of improved anticancer drugs. The effectiveness of these drugs is linked to the capability to selectively target cancer cells while minimizing toxicity to normal cells. As a result, specifically targeting potent drugs to cancer cells is a key to successful treatment of leukemia. There are various agents that have been investigated for targeted therapy including monoclonal antibodies against cell surface antigens, kinase inhibitors, signaling molecule inhibitors, and proteasome inhibitors (25). The use of antibodies for leukemic cell-targeted delivery of potent cytotoxic agents such as calicheamicin has also been investigated to overcome the dose limitations imposed by the side effects of non-targeted therapeutics (26).
Several targeted molecules are currently under intense investigation. CD33 is a glycoprotein up-regulated on normal myeloid cells during differentiation, which is expressed on the surface of leukemia cells in 85–90% of patients with acute myeloid leukemia(AML)(25, 27). Anti-CD33 antibody conjugated to calicheamicin has demonstrated response rates of 30–35% in adults and children with relapsed or primary refractory AML (25, 27). CD22 is a B-lymphoid lineage-specific glycoprotein that is highly expressed in B-cell lymphomas and B-precursor acute lymphoblastic leukemia(ALL)(25–26, 28). A CD22-specific immunoconjugate of calicheamicin has demonstrated preferential cytotoxic effects against CD22+ tumor targets and is more potent than that of unconjugated calicheamicin against CD22+ malignant cells (26). BCR-ABL kinase inhibitors (Imatinib and Dasatinib) were developed as highly specific small molecules for targeted therapy (29). Imatinib has potent clinical activity in chronic lymphoblastic leukemia, however, the response was short-lived (2–3 months) and some patients relapsed due to resistance to the drug (25, 29). The new class ABL/Src kinase inhibitor, Dasatinib, could potentially improve the activity of Imatinib. Dasatinib is highly effective as a single agent for patients with Imatinib-resistant chronic myelogenous leukemia(CML) with minimal side effects (25). Other targeted therapies include the inhibition of FMS-like tyrosine kinase 3(FLT3) which is expressed in more than 90% of AML patients (30). This proteasome inhibitor is part of a new class of drugs inhibiting the degradation of NF-κB, a transcriptional activator known to have anti-apoptotic activity. Mammalian Target of Rapamycin Complex-1 (mTOR), a serine/threonine kinase regulating cell growth, has been reported in more than 70% of AML patients (25). Inhibition of mTOR may be a useful therapeutic strategy for relapsed/refractory AML and ALL in children (25).
Actively targeting drugs using a drug carrier (e.g. polymer nanoparticle, liposome, dendrimer) may also differentially transport the therapeutically active molecule to the target cell. Molecular markers that are either uniquely expressed or overexpressed on leukemic cells may allow drugs or drug carriers to bind based on molecular recognition such as ligand-receptor or antigen-antibody interactions (30). On leukemic cells, several potential targets for drug carriers have emerged. For example, a current clinical trial is evaluating a peptide vaccination targeting different epitopes of the Wilms’ tumor gene 1 (WT1), the proteinase-3 derived epitope peptide (PR1), and the receptor for hyaluronic acid mediated motility (RHAMM/CD168) derived epitope R3 (30). Herceptin® was approved for women with breast cancer whose tumors have high level ofHER2 protein is the example of actively targeting drugs. Clinical experience with Herceptin® for the adjuvant treatment of HER2+ breast cancer began in 2000. In 2006, Herceptin® was approved for the adjuvant treatment of HER2+ breast cancer (31). Now, researchers are using Herceptin® to target potent chemotherapeutics or toxins for breast cancer chemotherapy. Some of the antibodies being explored for treating leukemia may soon follow similar ‘carrier’ approaches.
Recently, lymphocyte function-associated antigen-1 (LFA-1), a primary cell adhesion molecule, has also been investigated for targeting nanoparticles to human leukemic cell line (30, 32). Lima et al. reported that certain leukemic cells expressed 5-fold more LFA-1 than normal leukocytes (32). Since LFA-1 is expressed only on leukocytes, it may be possible to also use LFA-1 as a target for drug carrier conjugated with LFA-1 ligands and perhaps to differentially target certain types of leukemic cells.
In leukemia, LFA-1 expression determines the clinical and biological behavior of lymphoid malignancies. In human lymphomas, differential LFA-1 expression is related to the different maturation stage, growth pattern, degree of clinical aggressiveness, and anatomic site of tumor in different types of leukemia (33). Ahsmann et al. have found the presence of LFA-1 on tumor cells in low and medium grade malignant lymphomas but not on high grade malignant lymphomas(6). Since LFA-1 molecules play a role in cytolytic T cell-target cell conjugation, the absence of LFA-1 might contribute to an escape from immunosurveillance(6, 33–34). These trends suggest LFA-1 targeting may be preferred at earlier stages of cancer development.
Resting lymphocytes express the inactive form of LFA-1 but do not mediate firm adhesion to their ligands since the active form of LFA-1 can cause aggregation and clogging in blood vessels(10). The inactive form of LFA-1 is converted to the active conformational state by several stimuli such as chemokines and cytokines leading to a rapid increase in LFA-1 function(35). In leukemia, some patients’ leukemic cells may express inactive integrins that are non-functional, some that are constitutively active and some that are inactive but regulated by cytokines or other stimuli(35–36).
POTENTIAL OF LFA-1 TARGETED NANOPARTICLES
Targeted drug delivery systems promise to expand the efficacy and decrease the potential toxic side effects of drugs by increasing their localization to specific organs, tissues or cells (37). To enhance the targeting of nanoparticles to specific cells or tissues, target-specific ligands must be linked to the nanoparticle surface (38). For targeted delivery to tumors, the cellular target often consists of a membrane-bound tumor-associated antigen (TAA) such as folate receptor, which is amplified in a wide variety of human tumors (39). Target-specific ligands that are linked to nanoparticles have been divided into classes based on the target receptors: (i) targets that are preferentially expressed on endothelial cells of tumor blood vessels (e.g. integrin-αvβ3), (ii) targets that are overexpressed on tumor cells (e.g. HER2 and disialoganglioside (GD2)), and (iii) lineage-specific targets that are expressed at the same level on tumor cells and on normal cells (e.g. CD19)(40).
Cyclic peptides derived from the binding domain of ICAM-1, domain 1, with LFA-1 may be conjugated to drugs or drug carriers for targeting LFA-1. Cyclic peptides (cIBL, cIBR, cIBC, CH4 and CH7) derived from residues 1–21 of ICAM-1 Domain 1 (N terminus) have been investigated for the inhibition of ICAM-1/LFA-1 interaction between T-cells and epithelial cells (41). cIBR peptide, cyclo(1,12)Pen PRGGSVLVTGC, showed the highest affinity to the isolated LFA-1 receptor compared to cIBC and cIBL(42). cIBR peptide has also been shown to inhibit LFA-1/ICAM-1 interaction (43) and this peptide was internalized selectively in LFA-1 expressing T-cells (44). Recently, cIBR has been coupled to PLGA nanoparticles for targeting lymphoblastic leukemic T-cells (45). Here, the selective binding and internalization of cIBR peptide conjugated on PLGA nanoparticles were investigated in four different types of leukemic cell lines expressing different degree of LFA-1 expression. The specific binding of LFA-1 targeted nanoparticles was compared with untargeted nanoparticles. The specificity of this drug delivery system may be useful for treating certain types of leukemia typified by LFA-1 overexpression. Finally, these studies suggest that targeted nanoparticle therapies may represent a viable therapeutic approach for leukemia when considered within the context of current trends in targeted therapies reviewed here.
EXPERIMENTAL EVIDENCE OF LFA-1 TARGETED NANOPARTICLES
The correlation of LFA-1 expression and the binding degree of LFA-1 targeted nanoparticles were investigated as a promise for differently targeting leukemias typified by LFA-1 upregulation. First, PLGA nanoparticles were prepared by a solvent displacement method and cIBR peptide was conjugated on nanoparticles by carbodiimide chemistry (44). The mean diameter of NPs and cIBR-NPs were 178.9 ± 3.0 and 219.2 ± 10.1 nm, respectively (Table 1). A low polydispersity index was obtained from both formulations and indicated a narrow size distribution of the nanoparticle suspension. These colloids were consistently homogenous and stable. The zeta potentials of NPs and cIBR-NPs were observed to be −28.5 mV and −31.3 mV, respectively. The peptide density on the surface of nanoparticles after reaction was calculated assuming a normal Gussian particle size distribution (Table2).
Table 1.
Properties of nanoparticles.
| Property | Coumarin-6 loaded NP | Coumarin-6 loaded cIBR-NP |
|---|---|---|
| Mean size (nm) | 178.9 ± 3.0 | 219.2 ± 10.1 |
| Polydispersity | 0.099 ± 0.09 | 0.067 ± 0.06 |
| Zeta potential value (mV) | −28.5 ± 2.17 | −31.3 ± 1.37 |
Values are given as mean ± S.D. (n=3)
Table 2.
Calculated cIBR density on PLGA nanoparticles.
| Nanoparticle | Size (nm) | Total Surface Area(m2/g of PLGA) | Density of cIBR (pmol/cm2) |
|---|---|---|---|
| cIBR-NP | 219.2 | 0.0397 | 41.7 ± 2.4 |
Values are given as mean ± S.D. (n=3)
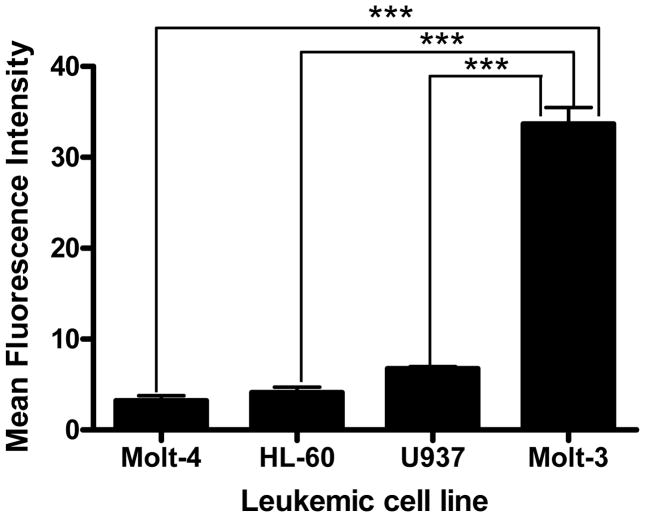
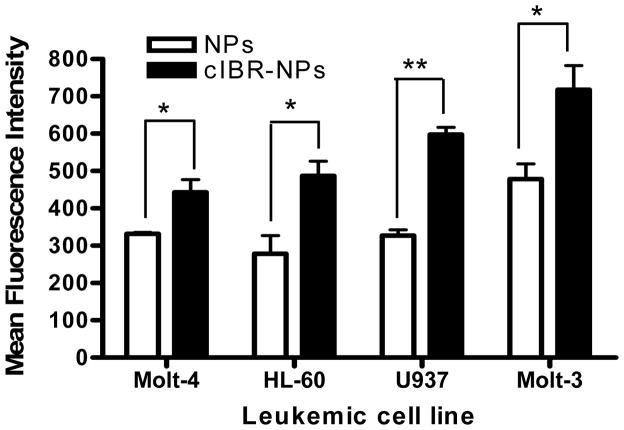
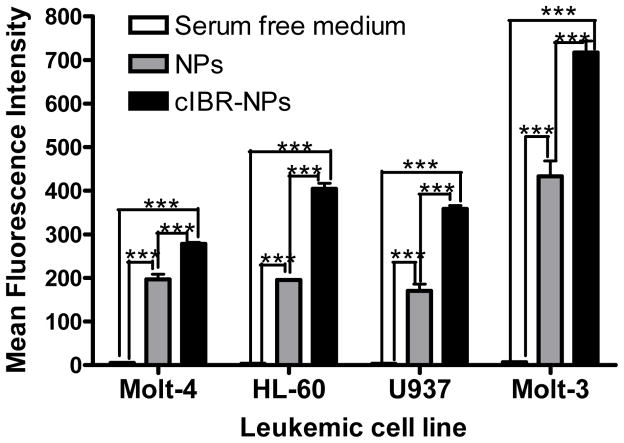
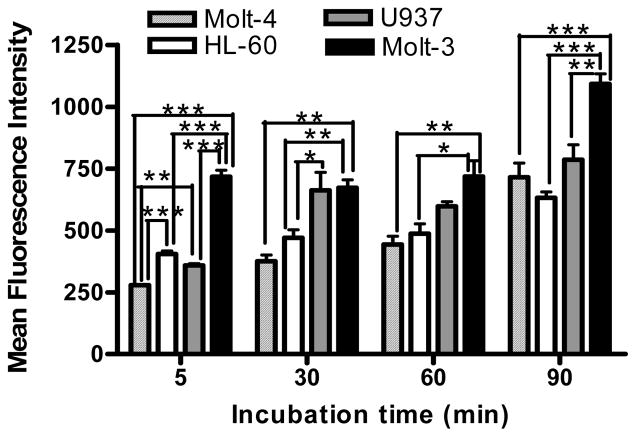
The relative expressions of LFA-1 on four different types of human leukemic cell lines were determined by immunofluorescence using anti-LFA-1-FITC antibody. The results indicated that Molt-3 cells (lymphoblastic cell line) expressed significantly more LFA-1 compared to the other cell types. U937 (promonocytic cell line), HL-60 (promyeloblastic cell line) and Molt-4 (lymphoblastic cell line) showed steadily decreasing LFA-1 expression levels (Fig.1). Then, cIBR-NPs and unconjugated NPs, both containing the fluorescent dye coumarin-6, were incubated with these cell lines and evaluated at several time intervals. Flow cytometry data indicated that the binding and internalization of cIBR-NPs in these four types of leukemic cell lines was significantly greater than untargeted NPs (p < 0.05 (Fig.2)), suggesting that cIBR was an effective targeting molecule when conjugated to a drug carrier. The binding of cIBR-NPs in these four types of leukemic cells was very fast and the fluorescence intensity of cIBR-NPs were significantly greater over time when compared to unconjugated NPs (Fig.3) .The rapid and specific binding of cIBR-NPs to LFA-1 on leukemic cells confirms previous reports (45). The uptake of cIBR-NPs in HL-60 and Molt-3 cells after 5, 30 and 60 minutes were similar (p > 0.05) but the fluorescence intensity increased with an incubation time of 90 min (p< 0.05 (Fig. 4)). In Molt-4, the fluorescence intensity generally increased slightly with incubation time (Fig. 4). After 30, 60 and 90 min, the binding of cIBR-NPs did not significantly increase in U937 suggesting that the cIBR-NPs may have saturated LFA-1 molecules. Beyond 30 min, the curves showed a plateau effect (Fig. 4). Molt-3 cells had the highest degree of binding and internalization of cIBR-NPs compared to HL-60 and Molt-4 cells at all time points (Fig. 4). Among the leukemic cells, Molt-3 cells showed the highest expression of LFA-1 in this study. The binding of cIBR-NPs in this cell line was also the highest (Fig. 4). Taken cumulatively, the increased degree of LFA-1 expression corresponded to the greater degree of binding and internalization of cIBR-NPs.
Fig. (1).
Relative expression of the adhesion molecule LFA-1 on leukemic cell lines. Data represent the mean ± S.D. from experiments performed in triplicate. *** indicates p< 0.001
Fig. (2).
Binding and internalization of cIBR-NPs and NPs after incubation (37°C; 60 min) with Molt-4, HL-60, U937 and Molt-3 cells. The fluorescence of leukemic cells incubated with cIBR-NPs were significantly greater when compared to unconjugated NPs. Results are given as mean ± S.D. (n=3); * indicates p< 0.05 and ** indicates p< 0.01
Fig. (3).
The comparison of fluorescence intensity between cells incubated with serum free medium (white color), cells incubated with NPs (gray color) and cells incubated with cIBR-NPs (black color) for 5 min incubation. Data are given as mean ± S.D.(n=3); **** indicates p< 0.0001
Fig. (4).
Kinetic binding profiles of cellular binding of cIBR-NPs by human leukemic cell lines (Molt-4, HL-60, U937 and Molt-3) after incubation for 5, 30, 60 and 90 min. Data represents mean ± S.D.(n=3); * indicates p<0.05, ** indicates p<0.01 and *** indicates p<0.001
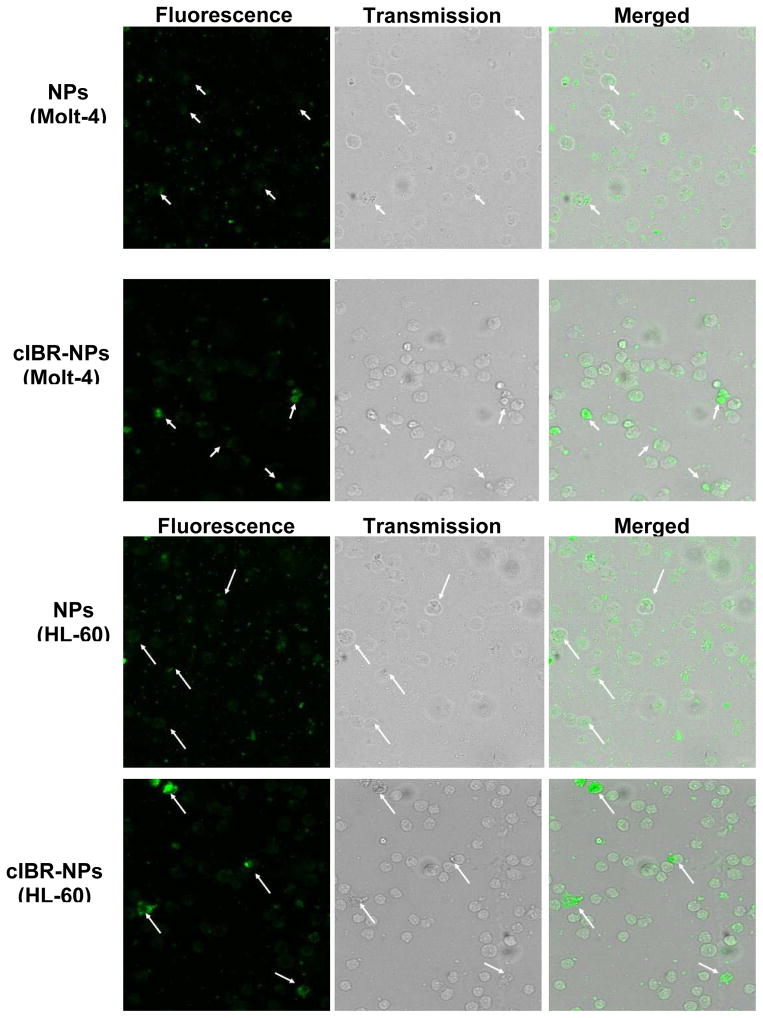
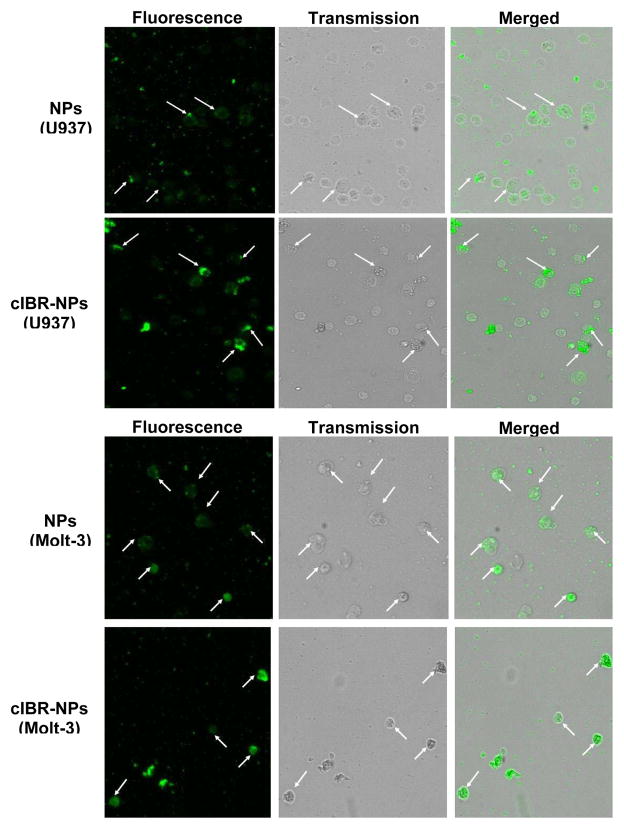
The cellular uptake of the coumarin-6 encapsulated cIBR-NPs and NPs were visualized by fluorescence microscopy. Fluorescence intensity was found to be greater for cIBR-NPs compared to untargeted NPs, further substantiating that cIBR-NPs interact with leukemia cells to a greater extent than untargeted nanoparticles. The results also indicated that the fluorescence intensity of Molt-3 cells, which had the highest level of LFA-1 expression, was greater than the intensity of Molt-4 cells, HL-60 cells and U937 cells incubated with cIBR-NPs (Fig.5). These results supported the cellular uptake of cIBR-NPs as determined by flow cytometry. Both analytical methods suggested that the level of LFA-1 expression corresponded to the degree of cellular uptake of cIBR-NPs.
Fig. (5).
Fluorescent micrographs of (A1) Molt-4 cells incubated with coumarin-6 loaded NPs, (A2) Molt-4 cells incubated with coumarin-6 loaded cIBR-NPs, (B1) HL-60 cells incubated with coumarin-6 loaded NPs, (B2) HL-60 cells incubated with coumarin-6 loaded cIBR-NPs, (C1) U937 cells incubated with coumarin-6 loaded NPs, (C2) U937 cells incubated with coumarin-6 loaded cIBR-NPs, (D1) Molt-3 cells incubated with coumarin-6 loaded NPs and (D2) Molt-3 cells incubated with coumarin-6 loaded cIBR-NPs. The white arrows indicate the binding of cIBR-NPs or NPs to leukemic cells. All images were captured at the magnitude of 10X and were normalized for variations in excitation light intensity.
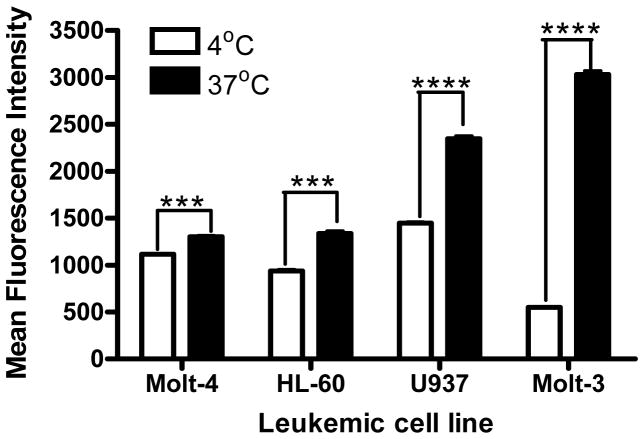
In order to evaluate the effect of temperature on the ability of leukemic cell lines to endocytose nanoparticles, cell uptake experiments were performed at different temperatures (4°C and 37°C). The cIBR-NP cell uptake at 37°C was obviously higher compared to 4°C(Fig. 6). The cIBR-NP uptake at 37°C were 1.2-,1.4-, 1.6- and 5.5-fold higher than at 4°C for Molt-4 cells, HL-60 cells, U937 cells and Molt-3 cells, respectively. The results suggested that the uptake of cIBR-NPs was somewhat temperature dependent indicating some involvement of an energy-dependent endocytic mechanism for nanoparticle internalization (46).
Fig. (6).
Effect of temperature on cellular binding and internalization of cIBR-NPs after 45 min incubation. Data are given as mean ± S.D.(n=3); *** indicates p<0.001 and **** indicates p<0.0001
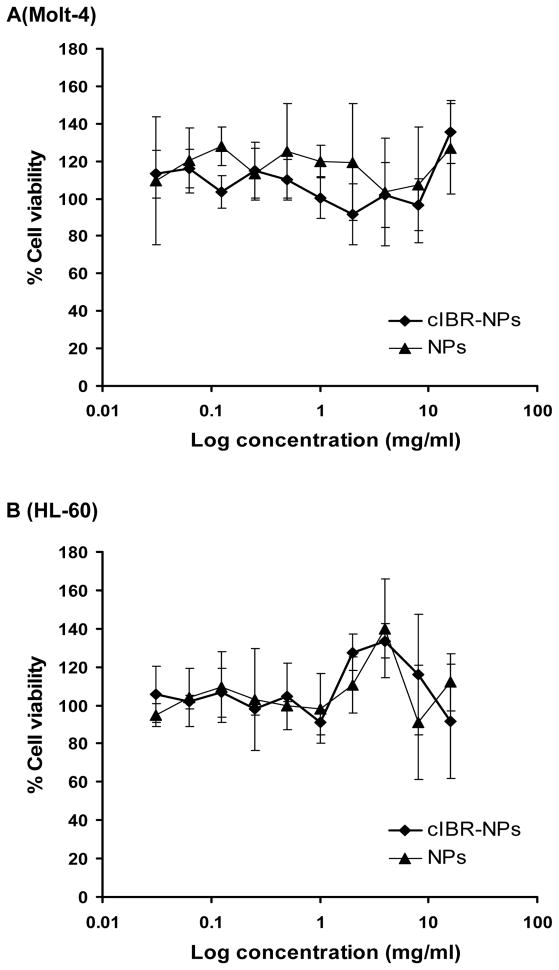
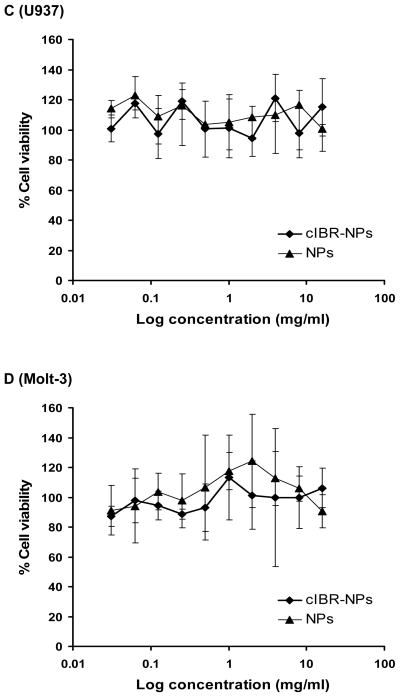
The cytotoxicities of cIBR-NPs and NPs were determined using an established MTS assay. The particle concentration was varied from 15.6 μg/ml to 8.0 mg/ml which included the concentration of nanoparticles used in the binding and internalization studies(2.2 mg/ml). The percentage of cell growth inhibition was assayed by quantifying surviving cells after 24 h incubation. Both nanoparticle formulations exhibited low cytotoxicity in the cell lines tested (Fig. 7). Results demonstrated that these drug carriers may be translatable due to low toxicity.
Fig. (7).
Cytotoxicity of cIBR-NPs and NPs in (A) Molt-4 cells, (B) HL-60 cells, (C) U937 cells and (D) Molt-3 cells.
In this study, the specific targeting of surface-modified nanoparticles conjugated with cIBR peptide was determined using four types of leukemic cell lines which exhibited dramatically different LFA-1 expression levels. The binding and uptake of cIBR-NPs was then compared. Particle size and size distribution are important characteristics of these types of nanoparticle systems. Desai et al. found that 100 nm nanoparticles had a 2.5 fold greater uptake than 1 μm microparticles, and 6 fold greater uptake than 10 μm microparticles in a Caco-2 cell line [33]. Others have also demonstrated that the smaller particles have relatively higher intracellular uptake. The particle size and size distribution (Table 1) confirmed that both cIBR and untargeted nanoparticles had small size and narrow size distribution. The surface charge of both nanoparticles were around −30 mV, which is sufficient to support NP stability since the surface charge prevents aggregation of particles (47).
Several leukemia cell lines were used to identify differential targeting of cIBR-NP as a function of LFA-1 expression levels. Molt-3 and Molt-4 cell lines were originally derived from a patient with T-cell acute lymphoblastic leukemia (ALL). HL-60 cell line was derived from a single patient with acute promyelocytic leukemia (AML) and U937 cell line was established from a human diffuse histiocytic lymphoma. Studies of LFA-1 expression on these cell lines showed that Molt-3 exhibited the highest level of LFA-1 expression. The uptake of targeted nanoparticles by Molt-4, HL-60 and Molt-3 cells were time dependent, however, it was hypothesized that cIBR–NPs quickly bound to cells via a saturable mechanism followed by internalization at 30 and 60 min incubation (48). At 90 min, increased binding and uptake may be facilitated by presentation of recycled LFA-1 receptor (49). The binding of cIBR-NPs did not significantly increase in U937 after 30 min, perhaps due to the differentiation of U937 monocytes after PMA stimulation (50). Others have reported differentiation of this cell line where PMA produced changes in morphology, certain cytochemical enzymes and the expression of macrophage markers (51).
The binding and internalization of both nanoparticles by leukemia cell line were confirmed via fluorescecent microscopy. Micrographs showed that cIBR-NPs bound better than untargeted nanoparticles. The results also indicated that the fluorescence intensity of Molt-3 cells, which had the highest level of LFA-1 expression, was greater than the intensity of Molt-4 cells, HL-60 cells and U937 cells incubated with cIBR-NPs. The very low fluorescent intensity of Molt-3 cells after incubation with cIBR-NPs at 4°C revealed that temperature has the most significant effect on the nanoparticle uptake of this cell line. To develop these types of targeted nanoparticles, identification of the different endocytic pathways involved will be critical. Additionally, the partial inhibition of nanoparticle uptake at low temperature (4°C) suggested that the internalization of the cIBR-NPs likely occurred via an energy dependent receptor-mediated endocytic process (45–46). Similar results have been reported when targeting ICAM-1 (52). The results of LFA-1 expression and cellular binding and internalization of nanoparticles revealed the relationship between the level of LFA-1 expression and the magnitude of nanoparticle binding and internalization. Molt-3 cells, which showed the highest LFA-1 expression, also exhibited the greatest binding and internalization of cIBR-NPs. Hence, certain leukemic cells overexpressing LFA-1 may differentially bind and internalize cIBR-NPs thus improving the selectivity of encapsulated chemotherapeutics.
CONCLUSION
Future leukemia therapeutics must offer improved drug selectivity to improve efficacy and reduce side effects of potent chemotherapies. The current state-of-art for targeting LFA-1 and other leukemia therapies were reviewed. Also, a brief study was presented to offer additional perspective regarding the potential use of nanoparticles for targeting potent leukemia drugs. cIBR-coupled PLGA nanoparticles targeting LFA-1 were studied to determine differential binding to four different types of leukemic cell line, Molt-4, HL-60, U937 and Molt-3 cells. The results of cellular uptake of cIBR-NPs from flow cytometry and fluorescence microscopy showed a significant increase in nanoparticle uptake in LFA-1 expressing cells compared to untargeted nanoparticles. Studies also revealed a strong correlation between LFA-1 expression level and the binding and internalization of cIBR-NPs. Moreover, the cIBR-NPs were non-cytotoxic as targeted drug carriers to LFA-1 expressing cells. These studies suggest that nanoparticles may be differentially targeted to surface markers on leukemic cells as a future targeted therapy.
EXPERIMENTAL PROCEDURES
Cell culture
Molt-3, Molt-4, HL-60 and U937 cell lines (American Type Culture Collection, VA, USA) were cultured in RPMI 1640 (Thermo Scientific HyQPAK, UT, USA) supplemented with 10%(v/v) fetal bovine serum and 1%(v/v) penicillin-streptomycin (10,000 U/ml), in a humidified 37°C incubator with 5% CO2 atmosphere.
Preparation of PLGA Nanoparticles loading fluorescent dye
Poly(DL-lactic-coglycolic acid)(50:50) with terminal carboxylate group (inherent viscosity 0.67dL/g, LACTEL Absorbable Polymers International, AL, USA) and coumarin-6 were dissolved in acetone and was slowly dropped into a stirred carboxylated Pluronic F-127 (BASF the Chemical Company, USA). The excess coumarin-6 and carboxylated Pluronic F-127 were removed by dialysis against 0.2% mannitol solution. The size and zeta potential of nanoparticles were evaluated using dynamic laser light scattering (ZetaPALS, Brookhaven Instrument Inc.).
Conjugation of cIBR Peptide to PLGA-Nanoparticles
A carbodiimde reaction was utilized for the coupling reaction of cIBR peptide to surface of nanoparticles using 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC, Thermo Fisher Scientific Inc., IL, USA) and N-hydroxysuccinimide(NHS, Thermo Fisher Scientific Inc., IL, USA) as coupling agents. The activated nanoparticles were coupled with cIBR peptides (170 μmole) for 12 h. To determine the density of cIBR peptide on NPs, unreacted peptide was quantified by gradient reverse phase HPLC (SHIMADZU) using a C18 column (Vydac® HPLC column Protein and Peptide C18 column).
LFA-1 Expression on Leukemic Cell Lines
Cells were treated with 0.4% Phorbol 12-myristate 13-acetate (PMA, Biomol International LP., PA, USA) for 48 h to stimulate LFA-1 on the cell membranes. AB serum were added in to the PMA activated leukemic cell lines (2×106 cells/ml) and incubated at 4°C for 10 min to block non–specific binding. Cells were then reacted with anti LFA-1-FITC at 4°C for 45 min and washed three times with cold 0.1% BSA in PBS. The fluorescent intensity was determined by using a flow cytometer.
In vitro Cellular Binding and Internalization of Nanoparticles
PMA treated Molt-3, Molt-4, HL-60 and U937 cell lines (2×105 cells/well) were incubated with cIBR -NPs or NPs (2.2 mg/ml) at 37°C for 5, 30, 60 and 90 min and washed three times with cold 0.1% BSA in PBS before measuring the fluorescent intensity by flow cytometer.
For microscopic study, cIBR-NPs or NPs (2.2 mg/ml) were incubated with each PMA pre-treated cell lines (2×106 cells/ml) for 30 min at 37°C, 5% CO2. A Nikon Eclipse 80i microscope equipped for epifluorescence was used for cell observation. Micrographs were taken using an Orca ER camera (Hamamatsu, Inc., Bridgewater, NJ) and analyzed by Metamorph, version 6.2 (Universal Imaging Corp., West Chester, PA).
Temperature Effect in Cellular Uptake of Nanoparticles
Four types of PMA treated leukemic cell lines (2×106 cells/ml) were incubated with cIBR-NPs or NPs (2.2 mg/ml) for 15, 30, 45, and 60 min at 4°C and 37°C and washed three times with ice-cold PBS. The uptake of cIBR-NPs or NPs by cells was determined by flow cytometry.
Cytotoxicity of Nanoparticles
Cytotoxicity of cIBR-NPs and NPs was evaluated on all studied cell lines using the MTS assay. Cells (8×103 cells/well/100μl) were incubated with various concentrations of cIBR-NPs or NPs for 24 h. After removing nanoparticles, fresh serum free RPMI 1640 and MTS were added. The plates were incubated for 4 h at 37°C. The quantity of formazan product was determined by the amount of 490 nm absorbance.
Acknowledgments
The authors would like to acknowledge funding from the Coulter Foundation and the Higuchi Biosciences Center, as well as additional funding from the American Heart Association. In addition, they would like to acknowledge funding from the NIH (R03 AR054035, P20 RR016443, and T32 GM08359-11) and the NSF (CHE 0719464). The authors acknowledge Prof. Jeffrey Krise for providing the fluorescence microscope. We would like to acknowledge funding support from Chiang Mai University and the Office of National Research Council of Thailand.
ABBREVIATIONS
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- cIBR-NPs
cIBR conjugated nanoparticles
- CML
Chronic myelogenous leukemia
- EDC
1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride
- FLT3
FMS-like tyrosine kinase 3
- GD2
Disialoganglioside
- HER2
Human Epidermal growth factor Receptor 2
- ICAM-1
Intercellular adhesion molecule-1
- LFA-1
Lymphocyte function-associated antigen-1
- mTOR
Mammalian Target of Rapamycin Complex-1
- NPs
Nanoparticles
- NHS
N-hydroxysuccinimide
- PLGA
Poly(DL-lactic-coglycolic acid
- PMA
Phorbol 12-myristate 13-acetate
- PML
Progressive leukoencephalopathy
- RHAMM/CD168
Receptor for hyaluronic acid mediated motility
- TAA
Tumor-associated antigen
- WT1
Wilms’ tumor gene 1
References
- 1.Giblin PA, Lemieux RM. LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics (Translated from eng) Curr Pharm Des. 2006;12(22):2771–2795. doi: 10.2174/138161206777947731. (in eng) [DOI] [PubMed] [Google Scholar]
- 2.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points (Translated from eng) J Cell Sci. 2003;116(Pt 23):4695–4705. doi: 10.1242/jcs.00876. (in eng) [DOI] [PubMed] [Google Scholar]
- 3.Kurzinger K, Reynolds T, Germain R, et al. A novel lymphocyte function-associated antigen (LFA-1): cellular distribution, quantitative expression, and structure. The Journal of Immunology. 1981;127(2):596. [PubMed] [Google Scholar]
- 4.Marlin S, Springer T. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 5.Wawryk SO, Novotny J, Wicks I, et al. The role of the LFA-1/ICAM-1 interaction in human leukocyte homing and adhesion (Translated from eng) Immunol Rev. 1989;108:135–161. doi: 10.1111/j.1600-065x.1989.tb00016.x. (in eng) [DOI] [PubMed] [Google Scholar]
- 6.Ahsmann EJ, Lokhorst HM, Dekker AW, Bloem AC. Lymphocyte function-associated antigen-1 expression on plasma cells correlates with tumor growth in multiple myeloma (Translated from eng) Blood. 1992;79(8):2068–2075. (in eng) [PubMed] [Google Scholar]
- 7.Sigal A, Novotny J, Wicks I, et al. The LFA-1 integrin supports rolling adhesions on ICAM-1 under physiological shear flow in a permissive cellular environment (Translated from eng) J Immunol. 2000;165(1):442–452. doi: 10.4049/jimmunol.165.1.442. (in eng) [DOI] [PubMed] [Google Scholar]
- 8.Hogg N, Clive L, Bates P, et al. Mechanisms contributing to the activity of integrins on leukocytes (Translated from eng) Immunol Rev. 2002;186:164–171. doi: 10.1034/j.1600-065x.2002.18614.x. (in eng) [DOI] [PubMed] [Google Scholar]
- 9.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) (Translated from eng) Cell. 1987;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. (in eng) [DOI] [PubMed] [Google Scholar]
- 10.Xingyuan M, Wenyun Z, Tianwen W. Leukocyte function-associated antigen-1: structure, function and application prospects (Translated from eng) Protein Pept Lett. 2006;13(4):397–400. doi: 10.2174/092986606775974429. (in eng) [DOI] [PubMed] [Google Scholar]
- 11.Guttman-Yassky E, Vugmeyster Y, Lowes M, et al. Blockade of CD11a by efalizumab in psoriasis patients induces a unique state of T-cell hyporesponsiveness (Translated from eng) J Invest Dermatol. 2008;128(5):1182–1191. doi: 10.1038/jid.2008.4. (in eng) [DOI] [PubMed] [Google Scholar]
- 12.Menter A. The status of biologic therapies in the treatment of moderate to severe psoriasis (Translated from eng) Cutis. 2009;84(4 Suppl):14–24. (in eng) [PubMed] [Google Scholar]
- 13.Kang X, Chen J, Qin Q, et al. Isatis tinctoria L. combined with co-stimulatory molecules blockade prolongs survival of cardiac allografts in alloantigen-primed mice. (Translated from eng) Transpl Immunol. 2010 doi: 10.1016/j.trim.2010.03.006. in Eng. [DOI] [PubMed] [Google Scholar]
- 14.Kvist M, Lemplesis V, Kanje M, et al. Immunomodulation by costimulation blockade inhibits rejection of nerve allografts (Translated from eng) J Peripher Nerv Syst. 2007;12(2):83–90. doi: 10.1111/j.1529-8027.2007.00126.x. (in eng) [DOI] [PubMed] [Google Scholar]
- 15.Arefanian H, Tredget EB, Rajotte RV, et al. Short-term administrations of a combination of anti-LFA-1 and anti-CD154 monoclonal antibodies induce tolerance to neonatal porcine islet xenografts in mice (Translated from eng) Diabetes. 2010;59(4):958–966. doi: 10.2337/db09-0413. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tredget EB, Arefanian H, Gill RG, Rajotte RV, Rayat GR. Monotherapy with anti-LFA-1 monoclonal antibody promotes long-term survival of rat islet xenografts (Translated from eng) Cell Transplant. 2008;17(6):599–608. doi: 10.3727/096368908786092757. (in eng) [DOI] [PubMed] [Google Scholar]
- 17.Bowles MJ, Pockley AG, Wood RF. Effect of anti-LFA-1 monoclonal antibody on rat small bowel allograft survival and circulating leukocyte populations (Translated from eng) Transpl Immunol. 2000;8(1):75–80. doi: 10.1016/s0966-3274(00)00007-1. (in eng) [DOI] [PubMed] [Google Scholar]
- 18.Navarini AA, Kerl K, French LE, Trueb RM. Control of Widespread Hypertrophic Lupus Erythematosus with T-Cell-Directed Biologic Efalizumab. (Translated from eng) Dermatology. 2010 doi: 10.1159/000277614. in Eng. [DOI] [PubMed] [Google Scholar]
- 19.Connolly MK, Kitchens EA, Chan B, Jardieu P, Wofsy D. Treatment of murine lupus with monoclonal antibodies to lymphocyte function-associated antigen-1: dose-dependent inhibition of autoantibody production and blockade of the immune response to therapy (Translated from eng) Clin Immunol Immunopathol. 1994;72(2):198–203. doi: 10.1006/clin.1994.1130. (in eng) [DOI] [PubMed] [Google Scholar]
- 20.Ninova D, Dean PG, Stegall MD. Immunomodulation through inhibition of multiple adhesion molecules generates resistance to autoimmune diabetes in NOD mice (Translated from eng) J Autoimmun. 2004;23(3):201–209. doi: 10.1016/j.jaut.2004.07.002. (in eng) [DOI] [PubMed] [Google Scholar]
- 21.Yagi N, Yokono K, Amano K, et al. Expression of intercellular adhesion molecule 1 on pancreatic beta-cells accelerates beta-cell destruction by cytotoxic T-cells in murine autoimmune diabetes (Translated from eng) Diabetes. 1995;44(7):744–752. doi: 10.2337/diab.44.7.744. (in eng) [DOI] [PubMed] [Google Scholar]
- 22.Willenborg DO, Staykova MA, Miyasaka M. Short term treatment with soluble neuroantigen and anti-CD11a (LFA-1) protects rats against autoimmune encephalomyelitis: treatment abrogates autoimmune disease but not autoimmunity (Translated from eng) J Immunol. 1996;157(5):1973–1980. (in eng) [PubMed] [Google Scholar]
- 23.The leukemia & Lymphoma Society. Leukemia, Lymphoma, Myeloma, Facts 2009–2010. 2009 Jul 01; Online http://www.leukemia-lymphoma.org/all_page?item_id=9346.
- 24.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58(2):71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 25.Brown P, Hunger SP, Smith FO, Carroll WL, Reaman GH. Novel targeted drug therapies for the treatment of childhood acute leukemia (Translated from eng) Expert Rev Hematol. 2009;2(9):145–158. doi: 10.1586/ehm.09.1. (in Eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia (Translated from eng) Leukemia. 2007;21(11):2240–2245. doi: 10.1038/sj.leu.2404866. (in eng) [DOI] [PubMed] [Google Scholar]
- 27.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules (Translated from eng) Blood. 1995;85(8):2005–2012. (in eng) [PubMed] [Google Scholar]
- 28.Boue DR, LeBien TW. Expression and structure of CD22 in acute leukemia (Translated from eng) Blood. 1988;71(5):1480–1486. (in eng) [PubMed] [Google Scholar]
- 29.Weisberg E, Manley P, Mestan J, et al. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL (Translated from eng) Br J Cancer. 2006;94(12):1765–1769. doi: 10.1038/sj.bjc.6603170. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carow CE, Levenstein M, Kaufmann S, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias (Translated from eng) Blood. 1996;87(3):1089–1096. (in eng) [PubMed] [Google Scholar]
- 31.Anonymous. Periodic Safety Update Report 2006 [Google Scholar]
- 32.Pyszniak AM, Welder CA, Takei F. Cell surface distribution of high-avidity LFA-1 detected by soluble ICAM-1-coated microspheres (Translated from eng) J Immunol. 1994;152(11):5241–5249. (in eng) [PubMed] [Google Scholar]
- 33.Stauder R, Greil R, Schulz T, et al. Expression of leucocyte function-associated antigen-1 and 7F7-antigen, an adhesion molecule related to intercellular adhesion molecule-1 (ICAM-1) in non-Hodgkin lymphomas and leukaemias: possible influence on growth pattern and leukaemic behaviour (Translated from eng) Clin Exp Immunol. 1989;77(2):234–238. (in eng) [PMC free article] [PubMed] [Google Scholar]
- 34.Hopkins AM, Baird AW, Nusrat A. ICAM-1: targeted docking for exogenous as well as endogenous ligands (Translated from eng) Adv Drug Deliv Rev. 2004;56(6):763–778. doi: 10.1016/j.addr.2003.10.043. (in eng) [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y. Activation of leukocyte function-associated antigen-1 on adult T-cell leukemia cells (Translated from eng) Leuk Lymphoma. 1999;36(1–2):15–23. doi: 10.3109/10428199909145945. (in eng) [DOI] [PubMed] [Google Scholar]
- 36.Winter SS, Sweatman J, Lawrence M, et al. Enhanced T-lineage acute lymphoblastic leukaemia cell survival on bone marrow stroma requires involvement of LFA-1 and ICAM-1 (Translated from eng) Br J Haematol. 2001;115(4):862–871. doi: 10.1046/j.1365-2141.2001.03182.x. (in eng) [DOI] [PubMed] [Google Scholar]
- 37.Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Advanced Drug Delivery Reviews. 2000;41(2):147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 38.Dinauer N, Balthasar S, Weber C, et al. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26(29):5898–5906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 39.Ni S, Stepson S, Lee R. Folate receptor targeted delivery of liposomal daunorubicin into tumor cells. Anticancer research. 2002;22(4):2131–2135. [PubMed] [Google Scholar]
- 40.Marcucci F, Lefoulon F. Active targeting with particulate drug carriers in tumor therapy: fundamentals and recent progress. Drug discovery today. 2004;9(5):219–228. doi: 10.1016/S1359-6446(03)02988-X. [DOI] [PubMed] [Google Scholar]
- 41.Anderson M, Siahaan T. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides(New York, NY 1980) 2003;24(3):487–501. doi: 10.1016/s0196-9781(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 42.Anderson M, Yakovleva T, Hu Y, Siahaan T. Inhibition of ICAM-1/LFA-1-mediated heterotypic T-cell adhesion to epithelial cells: design of ICAM-1 cyclic peptides. Bioorganic & Medicinal Chemistry Letters. 2004;14(6):1399–1402. doi: 10.1016/j.bmcl.2003.09.100. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman T, Oyarzabal J, Sebastian E, et al. ICAM-1 Peptide Inhibitors of T-cell Adhesion bind to the allosteric site of LFA-1. An NMR Characterization. Chemical Biology and Drug Design. 2007;70(4):347. doi: 10.1111/j.1747-0285.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 44.Anderson M, Siahaan T. Mechanism of binding and internalization of ICAM-1-derived cyclic peptides by LFA-1 on the surface of T cells: a potential method for targeted drug delivery. Pharmaceutical research. 2003;20(10):1523–1532. doi: 10.1023/a:1026188212126. [DOI] [PubMed] [Google Scholar]
- 45.Chittasupho C, Manikwar P, Krise J, Siahaan T, Berkland C. cIBR Effectively Targets Nanoparticles to LFA-1 on Acute Lymphoblastic T Cells. 2009 doi: 10.1021/mp900185u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain A, Jain S. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. European Journal of Pharmaceutical Sciences. 2008;35(5):404–416. doi: 10.1016/j.ejps.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Mohanraj V, Chen Y. Nanoparticles-A review. Tropical Journal of Pharmaceutical Research. 2007;5(1):561. [Google Scholar]
- 48.Jain A, Jain SK. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors (Translated from eng) Eur J Pharm Sci. 2008;35(5):404–416. doi: 10.1016/j.ejps.2008.08.008. (in eng) [DOI] [PubMed] [Google Scholar]
- 49.Fabbri M, Di Meglio S, Gagliani M, et al. Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the alphaL/beta2 integrin, LFA-1, during leukocyte chemotaxis (Translated from eng) Mol Biol Cell. 2005;16(12):5793–5803. doi: 10.1091/mbc.E05-05-0413. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenney D, Morahan P. Effects of differentiation of human macrophage-like U937 cells on intrinsic resistance to herpes simplex virus type 1. The Journal of Immunology. 1987;139(9):3076. [PubMed] [Google Scholar]
- 51.James S, Williams M, Newland A, Colston K. Leukemia Cell Differentiation Cellular and Molecular Interactions of Retinoids and Vitamin D. General pharmacology. 1999;32(1):143–154. doi: 10.1016/s0306-3623(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 52.Muro S, Gajewski C, Koval M, Muzykantov V. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]