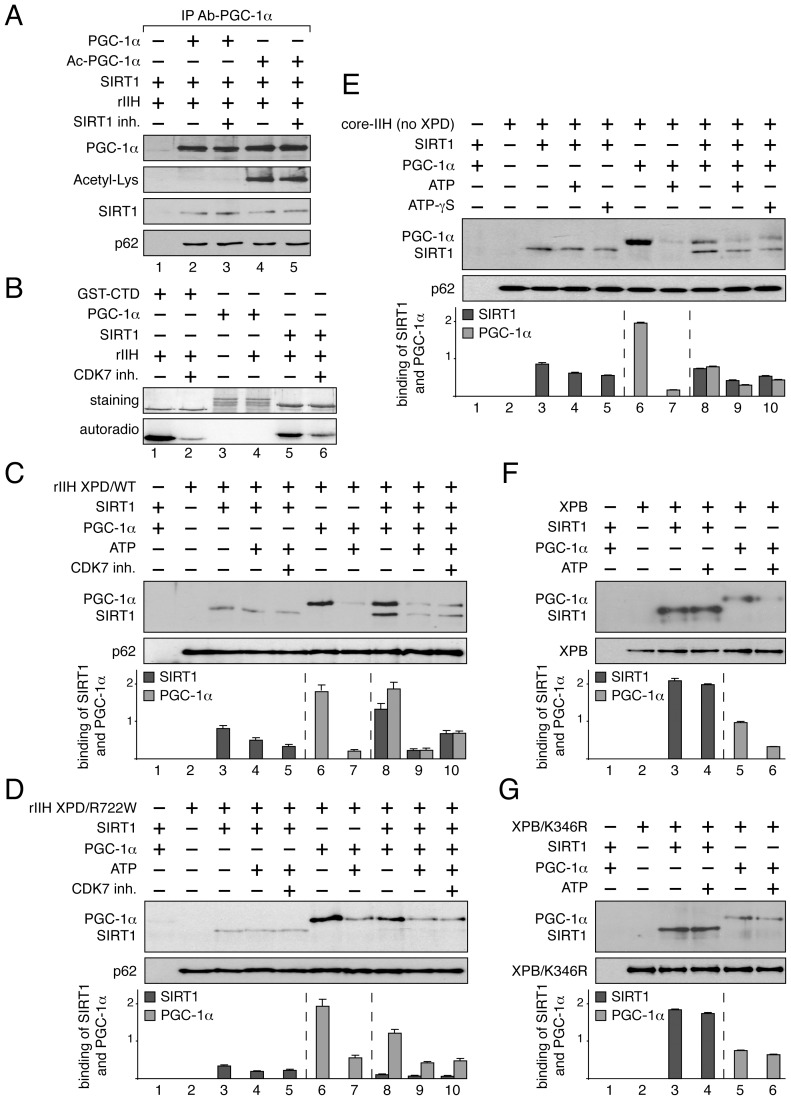
Figure 5. Dynamic partnership between TFIIH, PGC-1α and SIRT1.
(panel A) SIRT1 and TFIIH bind to PGC-1α independently to its acetylation status. After immunoprecipitation with specific antibodies (IP Ab-PGC-1α), non acetylated (PGC-1α, lanes 2–3) and acetylated PGC-1α (Ac-PGC-1α, lanes 4–5) were incubated with purified SIRT1 and recombinant TFIIH (rIIH). When indicated (+), specific SIRT1 inhibitor (SIRT1 inh., 10 µM) was also added. Co-immunoprecipitated proteins were visualized by western blots with anti-PGC-1α, -acetyl-Lysine, -p62 and -SIRT1 antibodies. (panel B) In vitro phosphorylation of SIRT1. When indicated (+), GST-C-terminal domain of the largest subunit of the RNA pol II (GST-CTD, 90 kDa) (used as positive control, lanes 1–2) [53], PGC-1α (130 kDa, lanes 3–4) and SIRT1 (110 kDa, lanes 5–6) were incubated with recombinant TFIIH (rIIH) in the presence of [γ-32P] ATP and CDK7 inhibitor (CDK7 inh.). Coomassie blue staining gel (top panels) and autoradiography (bottom panels) of the incubated fractions are shown. (panels C–G) When indicated (+), SIRT1 (110 kDa), PGC-1α(130 kDa, ATP (100 nM), CDK7 inhibitor (CDK7 inh.) and non-hydrolyzable ATP analog (ATP-γS, 100 nM) were incubated with either immunoprecipitated recombinant TFIIH with WT XPD subunit (rIIH XPD/WT, panel C), recombinant TFIIH with mutated XPD (rIIH XPD/R722W, panel D), core-TFIIH without XPD (panel E), XPB subunit (panel F) or mutated XPB bearing the point mutation K346R (XPB/K346R, panel G). Co-immunoprecipitated proteins were visualized by western blots using anti-SIRT1, -PGC-1α, -p62 and -XPB antibodies. Graphs depict the binding of PGC-1α and SIRT1.