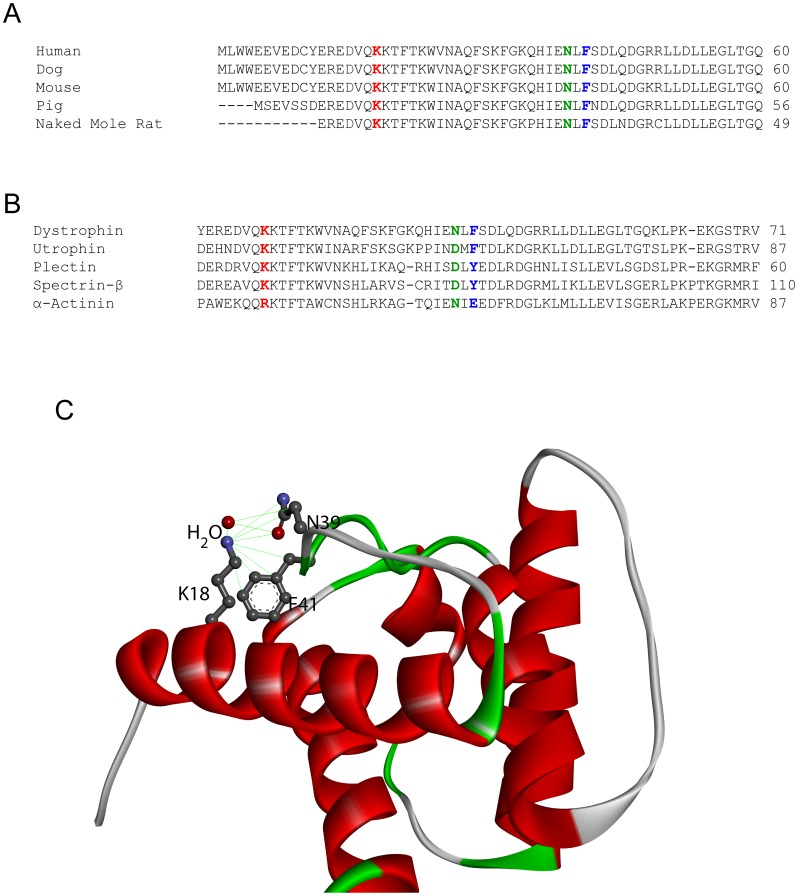
Figure 5. Sequence and structural analysis of the K18N mutation.
(A) Sequence alignment of dystrophin from various mammals. Lysine at the 18th position, asparagine at the 39th position, and phenyl alanine at the 41st position are shown in red, green, and blue colors respectively. (B) Sequence alignment of similar actin binding domains from other human proteins. Residues at the 18th, 39th, and 41st positions are shown in red, green, and blue colors respectively. (C) Structural view of stabilizing interactions formed by the sidechain of K18 in the WT dystrophin structure. All atoms that come close to the amide nitrogen of the lysine sidechain are shown by connecting green lines.