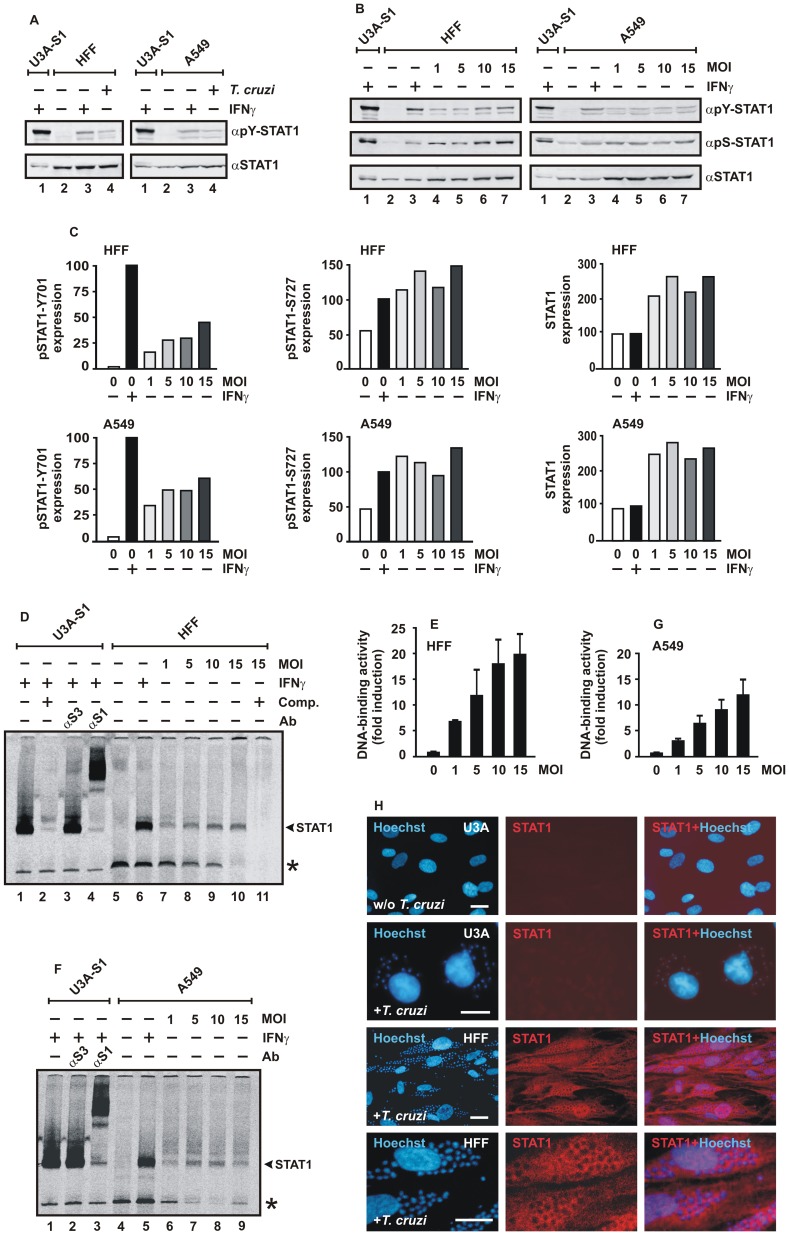
Figure 2. Parasitic infection with T. cruzi induces a sustained and cell type-independent activation of STAT1.
(A) Immunoblots demonstrating tyrosine phosphorylation and expression levels of STAT1 in differentially treated HFF (left) and A549 cells (right) using antibodies against tyrosine-phosphorylated STAT1 (αpY-STAT1) and, after stripping off of bound immunoreactivity from the membranes, pan-STAT1 antibody (αSTAT1). Cells were either left untreated (lane 2), stimulated for 18 h with 5 ng/ml IFNγ (lane 3) or challenged for 18 h with T. cruzi trypomastigotes at an MOI of 10 (lane 4). Cellular extracts from IFNγ-treated U3A cells (45 min) expressing recombinant STAT1 were used as control (lane 1) (n = 3). (B) Infectious dose-dependent increase in STAT1 tyrosine and serine phosphorylation in cytokine-unstimulated HFF (left) and A549 cells (right). Equal numbers of cells were exposed to parasites for 18 h at different MOIs. Representative immunoblotting experiments using antibodies specifically reacting with phospho-Y701- (αpY-STAT1), phospho-S727- (αpS-STAT1) and pan-STAT1 antibody (αSTAT1) are shown (n = 4). (C) Quantification of Western blot results for expression of phospho-Y701-, phospho-S727- and total STAT1 in extracts from cells infected with increasing doses of T. cruzi, as depicted in Figure 2B. Phosphorylation and expression levels were compared to the signal intensity in uninfected cells stimulated for 3 h with IFNγ set as 100. (D-G) T. cruzi infection elicits GAS-binding activity. Similar extracts as used for Western blotting (B) were incubated for 30 min with [33P]-labeled DNA containing a single STAT binding site (M67) and then loaded onto a polyacrylamide gel for detection by EMSA. (D,F) The band corresponding to M67-bound STAT1 dimers was identified by supershift with a STAT1-, but not STAT3 antibody (Ab) and, in addition, by competition with a 750-fold molar excess of unlabeled M67-DNA (Comp.). STAT1-M67 complexes are marked with an arrowhead, asterisks indicate an unspecific band. (E,G) The histograms demonstrate the M67-binding activity in cytokine-untreated HFF cells (E) and A549 cells (G) plotted against the infection dose (MOI). (H) Immunocytochemical staining of STAT1 in T. cruzi-infected HFF cells using anti-STAT1 antibody C-24 and Cy3-labeled secondary antibody. The fluorescence micrographs show the intracellular distribution of endogenous STAT1 in methanol-fixed, Hoechst-stained HFF cells and, in contrast, the lack of STAT1 expression in uninfected (w/o T. cruzi) and infected (+T. cruzi) U3A cells (scale bar 20 µm). Note that there was no co-localization of cytoplasmic STAT1 and Hoechst-stained amastigotes and that some parasite-containing cells showed nuclear accumulation of STAT1.