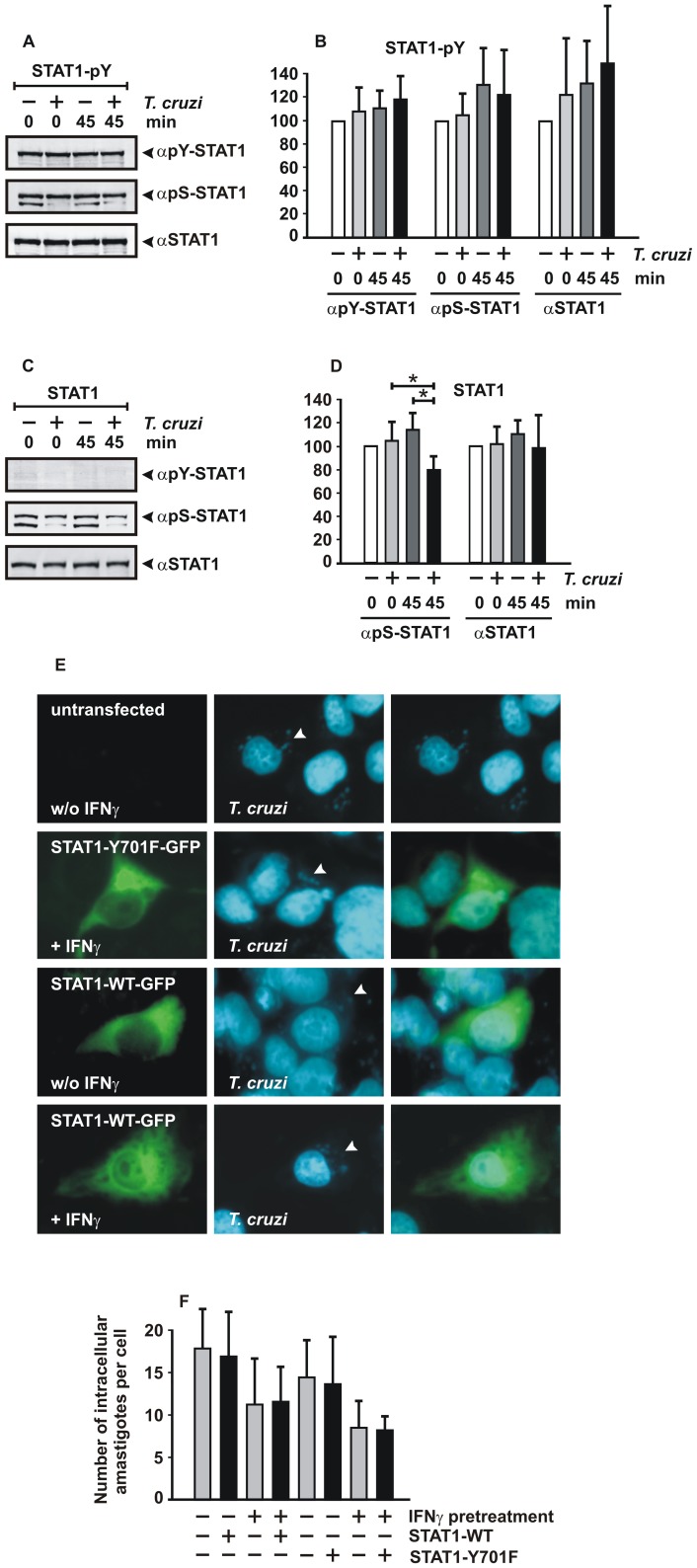
Figure 5. STAT1 serine phosphorylation is impaired in T. cruzi-infected cells.
In vitro dephosphorylation assays were performed by incubating equal amounts of cell extracts from STAT1-GFP-expressing U3A cells (20 µl) with cell extracts from uninfected or parasite-infected STAT1-negative U3A cells. Co-incubation of the lysates lasted for 0 min and 45 min at room temperature, respectively. Before cell lysis, the U3A cells expressing the marker protein STAT1-GFP had either been treated with IFNγ (A,B) or left untreated (C,D). The reactions were then loaded on SDS polyacrylamide gels and tested for the amount of phosphorylated and total STAT1 by means of Western blotting using the antibodies indicated. Representative Western blots (A,C) and a quantification of these results (B,D) are shown. Arrowheads at the right-hand margin of the membranes mark bands corresponding to STAT1. Note that the faster-migrating band labeled with the anti-phospho-S727 antibody is an unknown non-STAT1 protein, whose expression is typically reduced in extracts from parasite-infected cells. The experiment was repeated at least four times with similar results. (E) Detection of intracellular parasites in STAT1-reconstituted U3A cells. Cells expressing a GFP fusion of wild-type STAT1 or the tyrosine-phosphorylation-deficient point mutant Y701F were treated for 6 h with 5 ng/ml IFNγ or left untreated, as indicated. Subsequently, cells were infected for 18 h with parasites at an MOI of 2, and parasitic and human nuclei were stained in fixed cells with Hoechst dye. Adjacent non-transfected cells were used as control. Arrowheads mark localization of parasites in the cytoplasm of human cells. The experiment was performed twice for each condition with similar results. (F) Quantification of results from (E) in at least n = 14 cells per sample showing that STAT1-GFP expression did not prevent replication of amastigotes.