Figure 2. Overall structure of ZmaA-AT.
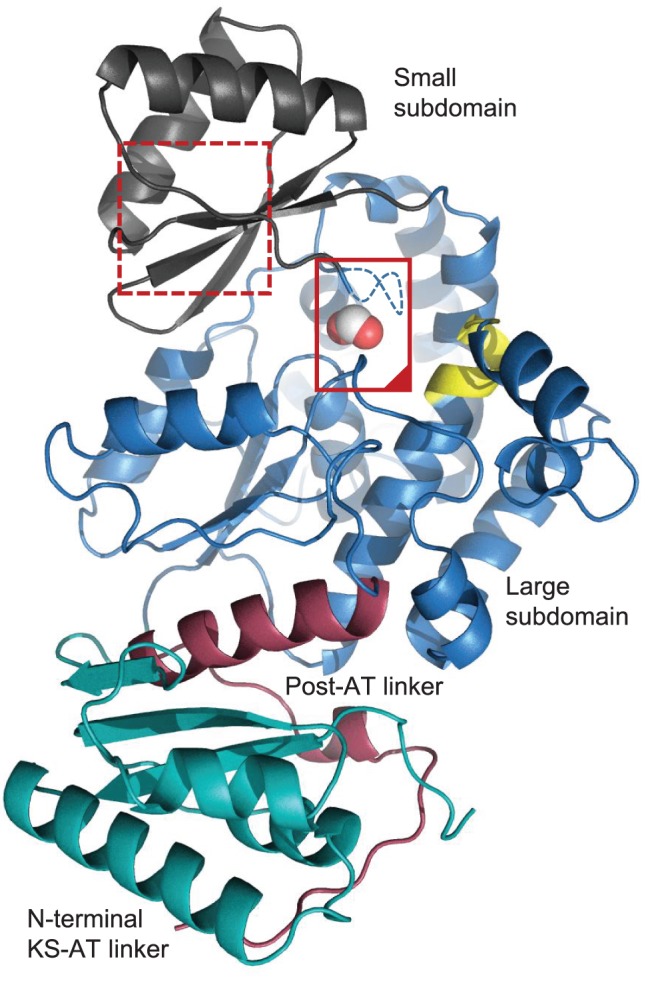
The N-terminal KS-AT linker (green), α/β-hydrolase large subdomain (blue), small subdomain (gray), and post-AT linker (red) make up the complete asymmetric unit. The active site of ZmaA-AT (inside solid red box) is bounded on the left by the substrate pocket lid (containing the YASH motif, which in ZmaA-AT is GAAH) and on the top by the RVDVVQ motif (yellow) and is occupied by formate (spheres); cf. Figure 3. Residues E293-G294-A295 are not observed and are indicated with a dashed line. The proposed substrate ACP binding surface M286-E293 contains the methionine residues of the RXR motif (MCM in ZmaAT) (dotted red box) which correspond to the inchoate β-strand of the ferredoxin fold in the smaller subdomain of other ATs; cf. Figure 4.
