Figure 2. Residue serine 243 is critical for the association of HPV-16 E2 with Brd4.
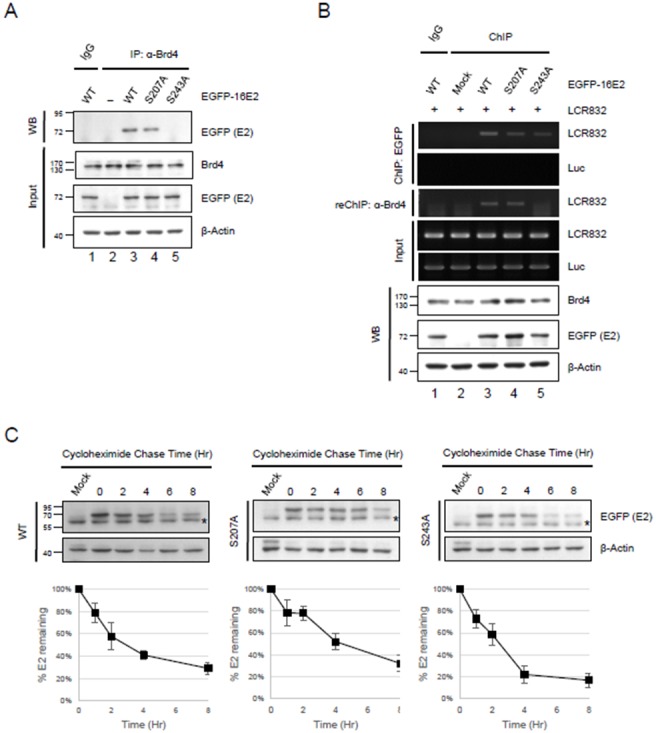
A) 293 cells were transfected with expression vectors for EGFP-tagged HPV-16 E2 or mutated E2. Cell extracts were immunoprecipitated with anti-Brd4 antibody or anti-IgG as a control in incubation buffer containing EtBr and the immunoblotting was performed with antibodies as indicated. B) C33A cells were cotransfected with HPV-16 LCR reporter and EGFP-tagged E2 or mutants. At 48 h posttransfection, chromatin extracts were immunoprecipitated with antibody against Brd4. The eluted DNA-protein complexes were subjected to PCR analysis (ChIP) or further immunoprecipitation with anti-Brd4 antibody (reChIP). C) The S243-mutated E2 protein (S243A) has a shorter half-life than the wild type. 293 cells were transfected with EGFP-tagged wild type or mutated E2. At 36 h posttransfection, the cells were treated with cycloheximide (50 µg/ml) for up to 8 h. Cell extracts were collected at the times shown and immunoblotted with the antibodies indicated. The graph below presents the quantified intensities of the bands. The expression levels of E2 were normalized using β-actin and the percentage of E2 at each time point relative to that of the control (without CHX treatment, set at 100) are shown. Data shown are means of results from three independent experiments (mean ± standard deviation; n = 3).
