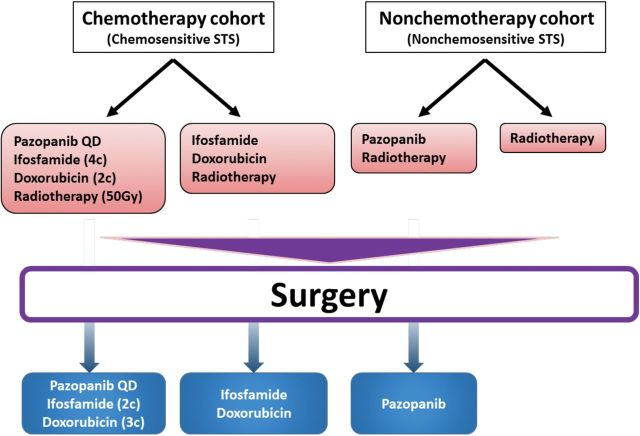
Figure 3.
A schema of the “Pazopanib Neoadjuvant Trial in Non-Rhabdomyosarcoma Soft Tissue Sarcomas” (PAZNTIS) (ARST1321). All patients will receive radiotherapy (50 Gy in 25 fractions) preoperatively. Neoadjuvant chemotherapy, which consists of four cycles of ifosfamide and two cycles of doxorubicin, will be offered to those who have soft-tissue sarcomas (STSs) that are sensitive to chemotherapy. Following trial entry, patients in each cohort will be randomly assigned to receive preoperative daily (QD) pazopanib in addition to their neoadjuvant treatments. After their definitive surgery, patients who received systemic preoperative treatments will receive six months of postoperative systemic therapy. STS = soft-tissue sarcoma.