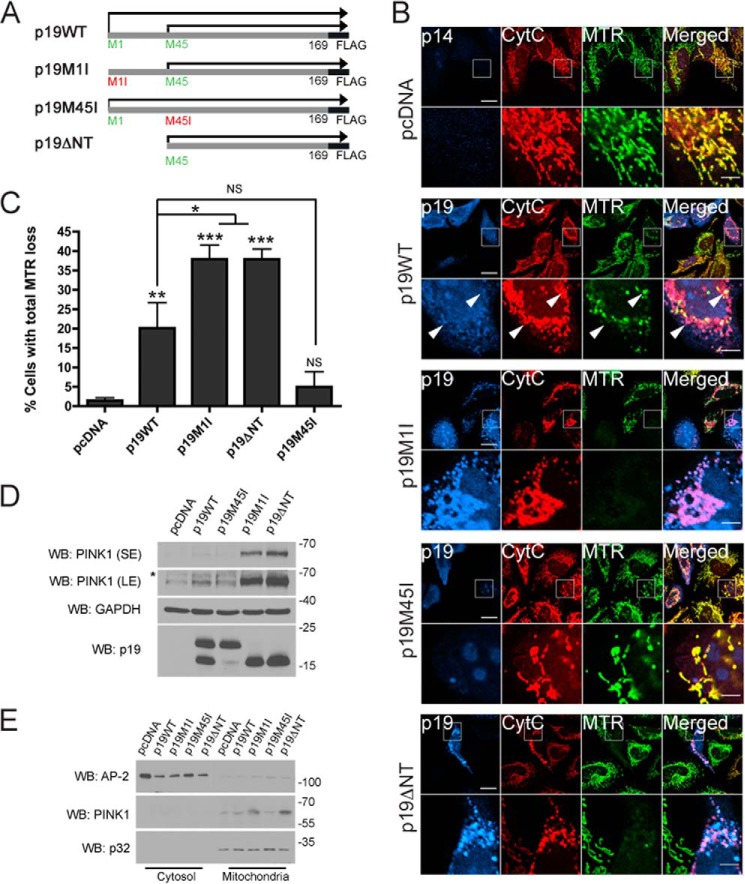
FIGURE 1.
smARF triggers loss of mitochondrial membrane potential and stabilizes PINK1. A, diagram of the p19ARF constructs used in this study: p19WT-FLAG, translated both from Met-1 and Met-45; p19M1I-FLAG, translated from Met-45 only; p19M45I-FLAG, translated from Met-1 only; and p19ΔNT-FLAG, which lacks the first 44 amino acids. B, HeLa cells expressing pcDNA, p19WT-FLAG (p19WT), p19M1I-FLAG (p19M1I), p19M45I-FLAG (p19M45I), or p19ΔNT-FLAG (p19ΔNT) for 24 h and stained with the mitochondrial membrane potential indicator MTR were processed for immunofluorescence against cytochrome c (CytC) and p19 or p14 antibodies. Scale bar, 20 μm for low power and 5 μm for zoom. Arrows, polarized and depolarized mitochondria in the same p19WT-transfected cell. C, the percentage of cells in B negative for MitoTracker Deep Red staining while retaining mitochondrial cytochrome c staining was measured. At least 50 cells were counted per condition per experiment. All columns are compared with pcDNA or with p19WT. n = 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, HeLa cells expressing pcDNA, p19WT, p19M1I, p19M45I, or p19ΔNT for 24 h were lysed, and 50 μg of protein lysate were separated by SDS-PAGE followed by immunoblotting against PINK1, GAPDH, and p19ARF. SE, short exposure; LE, long exposure. *, nonspecific band. E, cells treated as in D were fractionated into cytosolic and mitochondria-enriched fractions and processed for immunoblotting against PINK1, AP-2 (cytosolic), and p32 (mitochondrial) antibodies. WB, Western blot. Error bars, S.E.