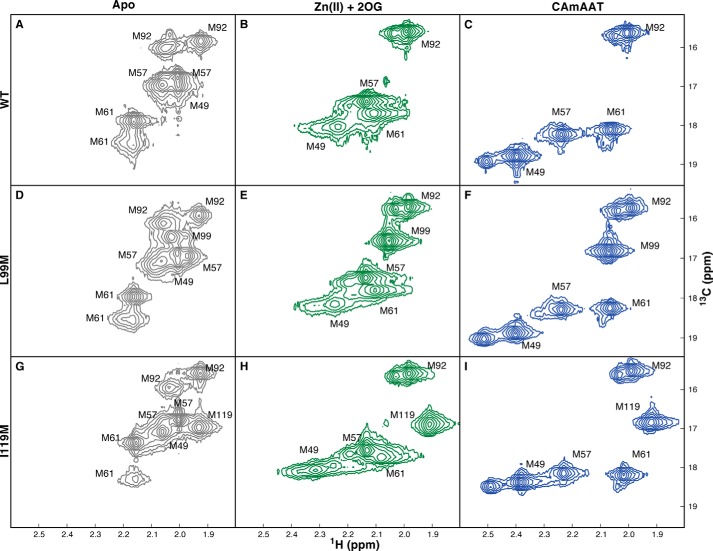
FIGURE 10.
NMR spectra of the L99M and I119M mutants verify that the conformation of the Fe(II)/2OG dioxygenase core is not significantly influenced by the conformational transition in the NRL. Two-dimensional spectra of WT (A–C), L99M (D–F), or I119M (G–I) enzymes selectively labeled with [13Cϵ]methionine were collected using a 1H-13C HSQC experiment from samples at 10 °C in 75 mm KCl, 50 mm NaH2PO4, pD 5.5. Unlabeled resonances in the Zn(II)/2OG and Zn(II)/2OG/5′-CAmAAT-3′ complexes are caused by the presence of trace metals, which have been verified not to affect resonance assignments. A, D, and G, spectra of the apoenzymes shown in gray. B, E, and H, spectra of the enzymes in the presence of 1.5× Zn(II) and 10× 2OG shown in green. C, F, and I. spectra of the enzymes in the presence of 1.5× Zn(II), 10× 2OG, and 1.5× 5′-CAmAAT-3′ shown in blue.