FIGURE 10.
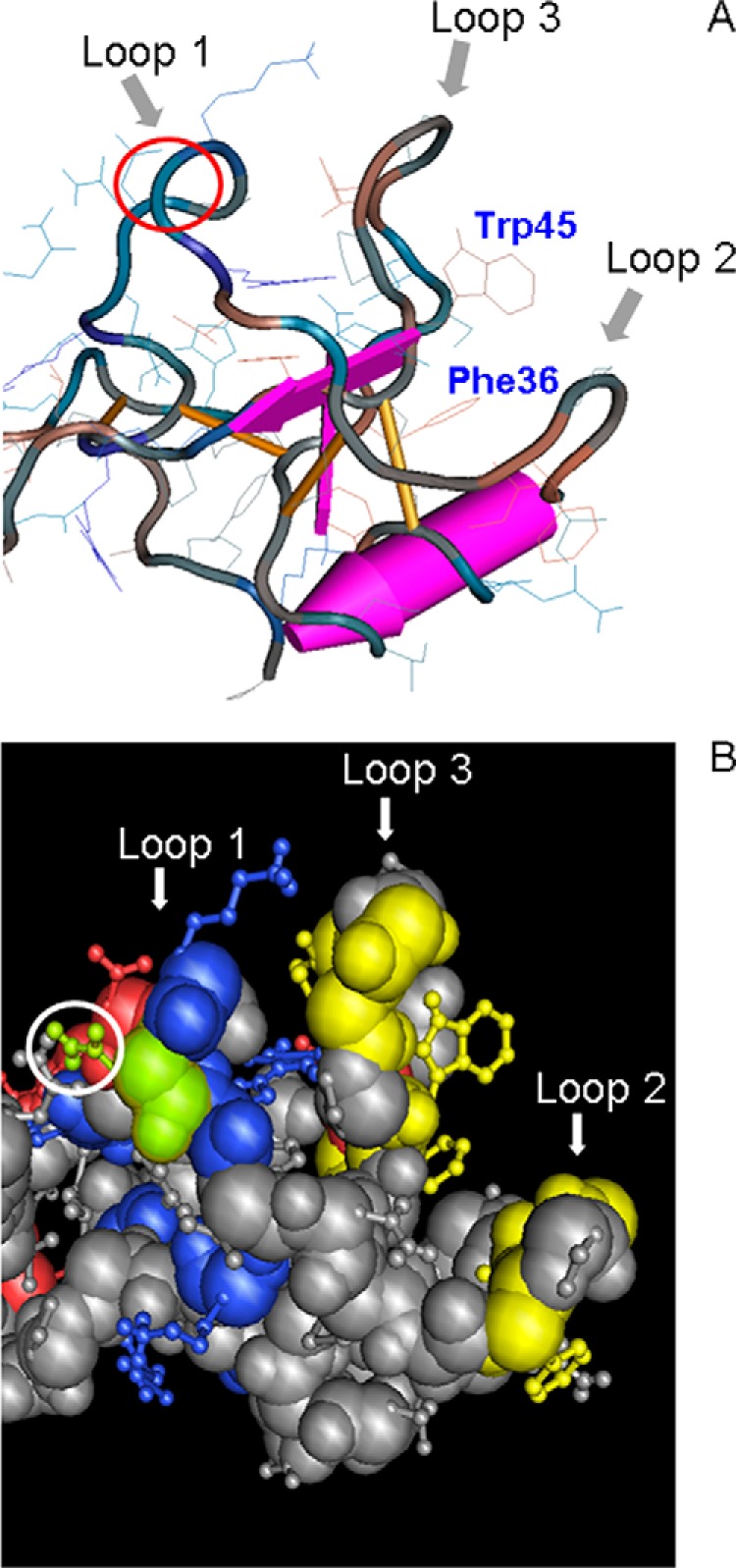
Three-dimensional view of porcine spasmolytic protein N-terminal domain P1. A, backbone conformation of pSP depicted using a worm diagram of domain I with toggled amino acid side chains (24). The three loops of the domain are indicated by arrows. The putative binding pocket presumably involved in protein-protein interaction is formed by loops 2 and 3 and characterized by highly conserved Phe-36 and Trp-45 (corresponding to Phe-59 and Trp-68). The amino acid position p15 in loop 1 corresponding to the N-glycosylation site in TFF2 is marked by a red circle. Amino acid numbering refers to the expressed protein. B, space-fill model of pSP (amino acid side chains as a ball-and-stick model) with marked charged residues (basic, blue; acidic, red), and green-highlighted Asn-15 (Asn-38) together with yellow-highlighted residues Phe-21, Gly-23, Ile-24, Phe-30, Phe-36, Val-40, Gly-42, Val-43, Trp-45 (corresponding to Phe-44, Gly-46, Ile-47, Phe-53, Phe-59, Val-63, Gly-65, Val-66, Trp-68), forming a hydrophobic patch. Amino acid numbering refers to the expressed protein (numbering in brackets to the SwissProt database entries).
