FIGURE 3.
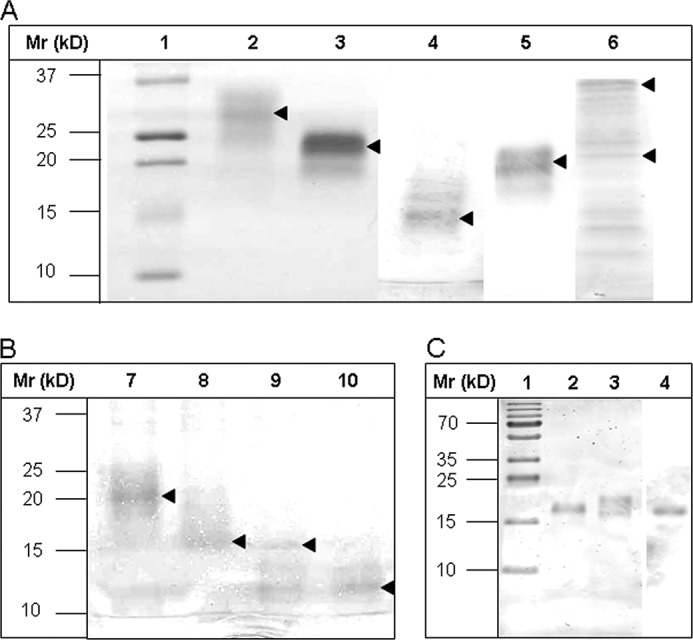
Gel electrophoretic separation of TFF2 probes before and after thrombin cleavage of strep-his-tags and Western blot of hTFF2-Fl with WFA after desialination/defucosylation. A and B, electrophoretic protein separation was performed in 5–20% gradient gels. A, Lanes 1–6 were loaded with samples: 1, molecular mass standard; 2, hTFF2-Fl; 3, N-P1; 4, C-P2; 5, C-P2*; 6, TFF2-Fl-(C52G). B, lanes were loaded with native or thrombin-treated probes N-P1 (lanes 7 and 8) or C-P2 (lanes 9 and 10). Proteins in A and B, lanes 2–10, were run in the native, non-reduced state. Arrows mark the positions of recombinant tagged or tag-free proteins. C, hTFF2-Fl probe was reduced and electrophoretically separated in a 15% polyacrylamide gel and either silver-stained or blotted onto PVDF membrane before staining with biotinylated lectin (WFA) and Strep-tactin-HRP. Lane 1, molecular mass standard; lane 2, chemically desialinated hTFF2-Fl (treatment with 0.1 m aqueous TFA at 80 °C for 1 h results also in partial defucosylation); lane 3, untreated hTFF2-Fl; lane 4, Western blot of hTFF2 with WFA. Lanes 1–3 show silver-stained proteins.
