FIGURE 1.
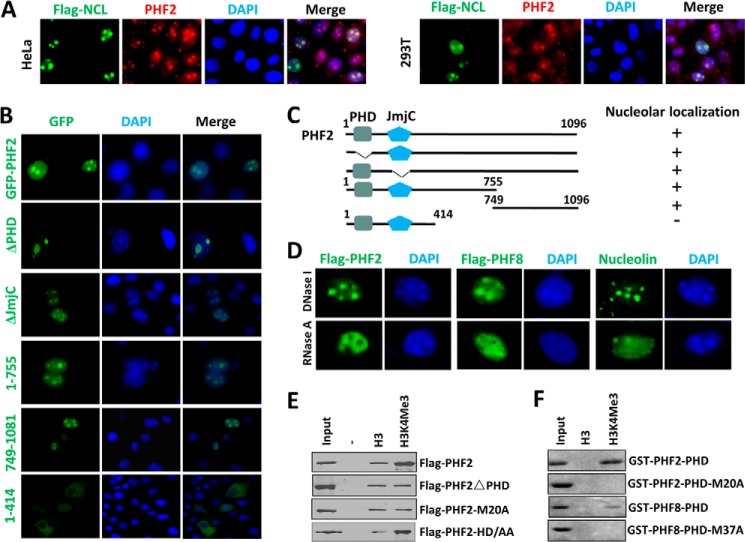
Characterization of PHF2 nucleolus localization. A, nucleolus localization for endogenous PHF2 in HeLa and 293T cells. HeLa or 293T cells were transfected with FLAG-tagged nucleolin (Flag-NCL), and double immunostaining was performed for FLAG tag and endogenous PHF2. B, the mapping of sequence determinant(s) for PHF2 nucleolus localization. HeLa cells were transiently transfected with GFP-tagged PHF2 and various deletion mutants, and their subcellular localization was determined by using a fluorescent microscope. Nuclei were stained with Hoechst 33342. C, diagram illustrating the various PHF2 deletion constructs used in B and a summary of their nucleolus localization status. D, the nucleolus localization of both PHF2 and PHF8 is sensitive to RNase A treatment but insensitive to DNase I treatment. The HeLa cells were transfected with either FLAG-PHF2 or FLAG-PHF8 and then treated with RNase A or DNase I at a final concentration of 100 μg/ml or 10 units/ml at 37 °C for 30 min before they were processed for immunofluorescent staining for FLAG-PHF2 or FLAG-PHF8 using anti-FLAG antibody. Immunofluorescent staining was also performed for endogenous nucleolin, which served as a control for nucleolus integrity. E, in vitro peptide pull-down assay examining the binding of FLAG-tagged PHF2, PHF2 with deletion of PHD domain (PHF2ΔPHD), and PHF2 with M20A mutation to control H3 and H3 peptide with H3K4me3. Both peptides contain the H3 N-terminal tail aa 1–21 with a C-terminal biotin moiety. F, in vitro peptide pull-down as in E but with purified recombinant GST-PHF2-PHD domain or GST-PHF8-PHD domain and their mutant as indicated.