FIGURE 4.
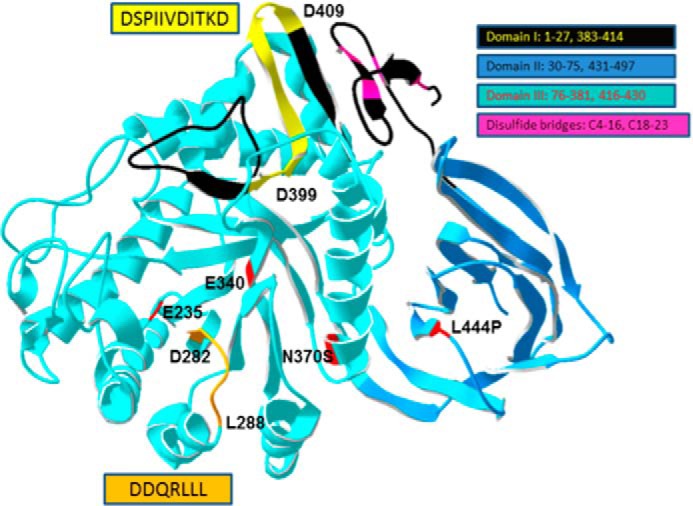
Structure of WT GCase highlighting the potential ldLIMP2 binding sequence localization. The two amino acid sequences from Fig. 3 were mapped to the crystal structure of WT human GCase. The orientation of the sequences is indicated by the amino acid numbers in the highlighted (yellow or orange) regions. The domains of GCase are shown in various indicated colors as are the disulfides. The potential ldLIMP-2 binding (yellow, Asp399–Asp409) sequence forms a surface-accessible loop in domain I (black, with the motif in yellow). The DSPIIVDITKD sequence and the DDQRLLL (orange, Asp282–Leu288 in Domain III) sequence are highly conserved in GCases from insects to humans (data not shown). The acid-base (Glu235) and nucleophile (Glu340) in catalysis and the N370S and L444P common mutations causal to Gaucher disease are shown in red (5).
