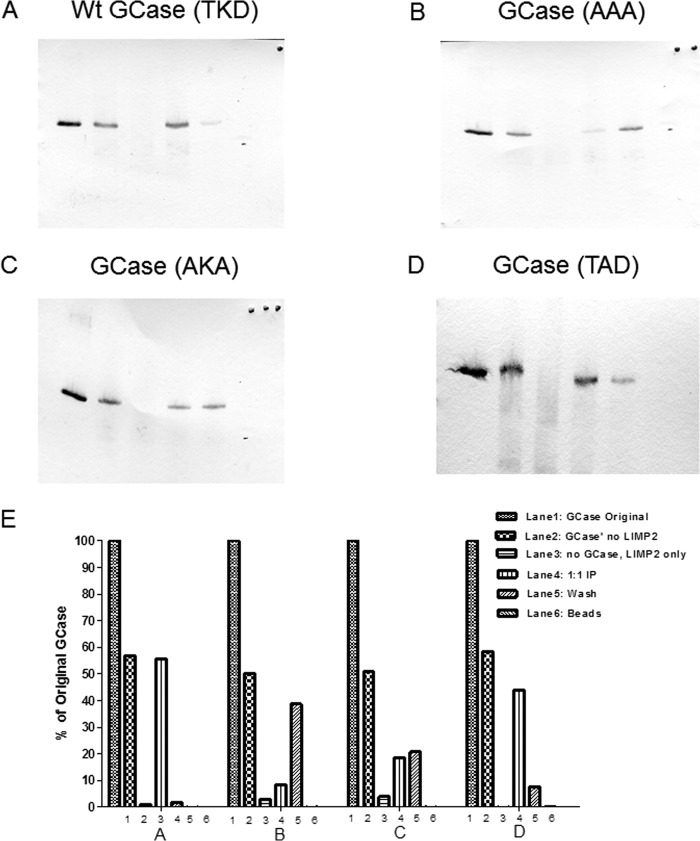
FIGURE 6.
Immunoprecipitation of the alanine-substituted GCases in the TKD sequence with ldLIMP-2. In A–D, lanes 1–6 correspond to the band densities indicated by the bars in E. The panels show that the WT sequence (A) had nearly complete binding with ldLIMP-2 (i.e. retention on the beads to which ldLIMP-2 was bound (lane 4) and no GCase in the wash (lane 5)). In comparison, the triple alanine mutant (B) shows essentially no binding to ldLIMP-2 (i.e. all of the GCase is in the wash (lane 5)). The AKA GCase mutant (C) showed about equal amounts of GCase in lanes 4 and 5, or about 50% binding to ldLIMP-2. The AKD GCase mutant (D) had a binding pattern that was similar to the WT sequence but with more GCase in the wash (lane 5) (i.e. somewhat less binding). The quantitative results are shown in E, in which 1, 2, 3, and 4 correspond to WT, AAA, AKA, and AKD, respectively. The results are typical of multiple experiments.