FIGURE 7.
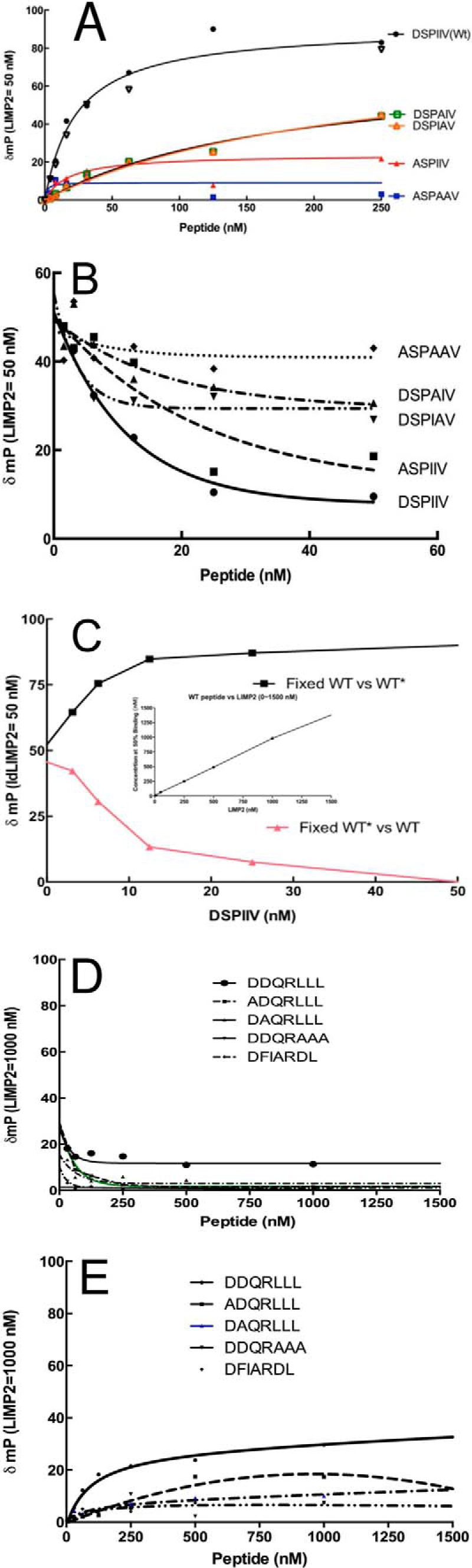
Binding and competition of fluorescence-labeled or unlabeled DSPIIV GCase peptides to ldLIMP-2. The change (δmP) in fluorescence polarization is plotted on the ordinate, and the increasing concentrations of the various peptides are shown on the abscissa. A, the concentration of ldLIMP-2 (50 nm) was fixed. With the WT (DSPIIV) peptide (● and ▿, duplicate experiments), saturation kinetics with about half-maximal binding to ldLIMP-2 was observed at 50 nm. The DSPAIV and DSPIAV mutants did not show saturation up to 250 nm, indicating their poor interaction with ldLIMP-2. The ASPAAP peptide showed background changes. The ASPIIV peptide showed δmP values slightly above background, indicating little binding to ldLIMP-2. B, to ensure that the label did not interfere/promote binding, similar studies were conducted using fluorescently labeled peptides as binders and their respective unlabeled peptides as competitors. Each of the unlabeled peptides “competed” with the labeled peptides in the expected ratios. C, labeled (WT*) and unlabeled (WT) peptides were used in complementary competition studies. Either the labeled or unlabeled WT peptides equally competed for binding to ldLIMP-2, showing that both were equally effective in binding and that the label did not change the properties of the peptide-ldLIMP-2 interaction. The inset indicates 1:1 stoichiometry and tight binding properties of DSPIIV and ldLIMP-2. D, the δmP of the WT (DDQRLLL) or variously alanine-substituted labeled peptides were plotted against the corresponding unlabeled peptide competitors. Note that the fixed ldLIMP-2 concentration was 1000 nm, or 20 times that used in Fig. 7, A and B. For all peptides, the δmP values were near or at background levels. E, binding δmP for the various peptides as in D. The signals were near background levels for all peptides using concentrations 5–10 times that for DSPIIV and with a 20 times greater LIMP-2 fixed concentration.
