FIGURE 9.
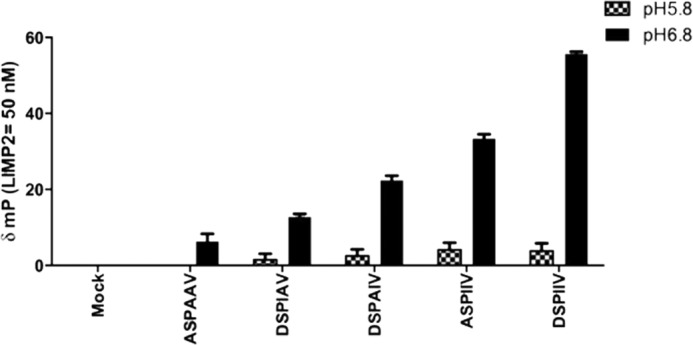
Effect of pH on binding WT and alanine-substituted DSPIIV peptides to ldLIMP-2. The binding of the different peptides to ldLIMP-2 showed little effect of alanine substitutions at pH 5.8. In comparison, incremental decreases were evident in ldLIMP-2 binding at pH 6.8, the approximate pH of the ER/cis-Golgi, as follows: the triple mutant (ASPAAV) having the lowest binding (<10% of WT), the DSPAIV (I402A) and DSPIAV (I403A) mutants being intermediate (∼40–50% of WT), and ASPIIV (D399A) having the least change (∼50–60% of WT), relative to WT. Error bars, S.E.
