Fig. 2.
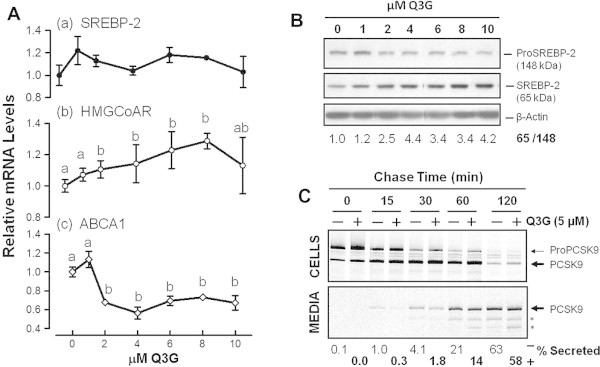
Q3G stimulates nuclear SREBP-2 production and delays PCSK9 secretion. Cells were incubated for 24 h in medium containing the indicated concentrations of Q3G. (A) qRT-PCR for mRNA levels of SREBP-2, HMGCoAR and ABCA1. Values are means of triplicate experiments ± SEM. They are expressed relative to untreated cells. Significant difference (P < 0.05) is represented in the graph by different letters above symbols of means ± SEM. (B) Sq-immunoblotting for cellular SREBP-2-related proteins. Density ratios of the 65-kDa SREBP over the 148-kDa precursor SREBP after normalization for β-actin were derived from two separate experiments. (C) Cells were pre-incubated for 24 h in medium 5 μM Q3G. After metabolic labeling with radioactive amino acids, labeled proteins were chased in Q3G-free non-radioactive medium, for varying lengths of time. PCSK9-related proteins were immunoprecipitated, fractionated by SDS–PAGE, and quantified by phosphorimaging. Upper panel: PCSK9-related proteins in cell lysates. Lower panel: PCSK9-related proteins in spent media. The percents of secreted PCSK9 were based on densitometric values of intracellular and extracellular bands corresponding to proPCSK9 and PCSK9. Asterisks (∗) indicate possible products of further PCSK9 processing.
