Abstract
The inner ear vasculature is responsible for maintenance of the blood-labyrinth barrier, transport of systemic hormones for ion homeostasis, and supplying nutrients for metabolic functions. Unfortunately, these blood vessels also expose the ear to circulating inflammatory factors resulting from systemic diseases. Thus, although the inner ear blood vessels are critical for normal function, they also facilitate pathological mechanisms that result in hearing and vestibular dysfunction. Despite these numerous critical roles of inner ear vasculature, little is known of its normal homeostatic functions and how these are compromised in disease. The objective of this review is to discuss the current concepts of vascular biology, how blood vessels naturally respond to circulating inflammatory factors, and how such mechanisms of vascular pathophysiology may cause hearing loss.
Keywords: blood-labyrinth barrier, blood vessels, glycocalyx, immunopathology, hearing loss
Learning Outcomes: As a result of this activity, the participant will be able to (1) identify regions in the cochlea that are susceptible to damage by inflammatory factors and (2) describe how inflammatory processes in the cochlea contribute to hearing loss.
The vasculature of the inner ear plays an important role in hearing and is dynamically responsive to certain insults.1 Control of ion and water homeostasis depends on vascular integrity to maintain the blood-labyrinth barrier. Meanwhile, the blood vessels carry immune cells, inflammatory factors, and hormones that can affect the function of the ear. When one considers the systemic delivery of steroids and other therapeutic drugs for hearing loss, the vasculature becomes an even more critical conduit and moderator of cochlear function. Despite the significant role of cochlear vasculature in health and disease, little is known of the mechanisms potentially involved in these processes. The goal of this review is to describe recent research in vascular pathophysiology and its involvement in hearing disorders.
Vascular Pathophysiology
Current vascular biology studies have established how circulating immune cells, antibodies, cytokines, and pathogens impact blood vessels. The endothelial cells that line the capillaries have a glycocalyx covering their luminal surface (Fig. 1). This glycocalyx is made up of transmembrane proteoglycan cores with glycosaminoglycan side chains, such as heparin sulfate and chondroitin sulfate.2 3 4 5 The glycocalyx serves as a barrier to prevent circulating immune cells and large molecules in the serum from reaching the endothelial cell surface.6 7 However, as long as this barrier is intact, there can be no movement of inflammatory factors into the tissue surrounding the capillaries if there is an injury or infection. Thus, although the glycocalyx serves as a natural homeostatic barrier to protect the tissues, it also has to be removed to facilitate the normal inflammatory events that are required to fight disease.
Figure 1.
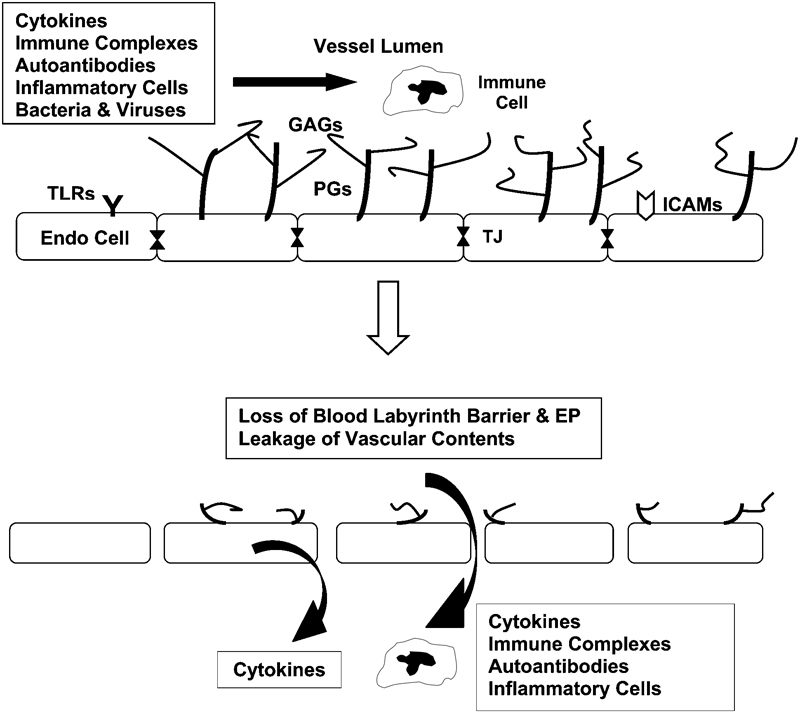
Vascular pathophysiology in response to circulating inflammatory factors. (Top) The glycocalyx is made up of transmembrane proteoglycan (PG) cores with glycosaminoglycan (GAG) side chains. This glycocalyx keeps red blood cells and immune cells in the central part of the capillary lumen and away from toll-like receptors (TLR) and intercellular adhesion molecules (ICAM) on the surface of endothelial cells. Endothelial cell (Endo cell) tight junctions (TJ) keep vascular components out of the pericapillary space. (Bottom) Elevated levels of circulating inflammatory factors strip off the glycocalyx and break down the tight junctions to permit movement of inflammatory factors into the surrounding tissues. Endothelial cells also produce inflammatory cytokines as part of their inflammatory response. The loss of tight junctions in the ear opens the blood-labyrinth barrier, causing compromised endolymph production and decreased endolymphatic potential (EP).
Various systemic inflammatory and infectious diseases elevate the circulating levels of immune factors, which include autoantibodies in the case of autoimmune diseases, bacterial, fungal, and viral components during infections, and inflammatory cytokines and chemokines that are elevated as a result of these conditions. Some of these cytokines strip off the protective glycocalyx, which exposes the endothelial cell surface to the circulating immune factors and initiates several events (Fig. 1).8 First, the endothelial cell itself produces its own inflammatory mediators, such as cytokines and chemokines, and releases them into the surrounding tissues.3 Endothelial cells also produce adhesion molecules on their surface that provide attachment sites for inflammatory cells that need to move across the vascular barrier.9 10 Some of these intercellular adhesion molecules are already on the surface and are uncovered when the glycocalyx is removed.11 Last, endothelial cells down-regulate their production of tight junction proteins (claudins, occludins), permitting movement of serum factors and inflammatory cells (macrophages, T-cells, etc.) through the intercellular spaces into the extracapillary space.12 13 14 Bacterial and viral infections also will cause this vascular reaction via various toll-like receptors that line endothelial cells, providing a mechanism for pathogens to elicit the same reaction by capillaries as part of the innate immune response. Thus, the endothelial cell is not a passive bystander, but rather an active participant in the natural immune response.
Although numerous inflammatory cytokines are increased in inflammatory disorders, the most problematic are interleukin-1, interleukin-6, and tumor necrosis factor-α (TNFα),14 15 because they are known to quickly strip the glycocalyx and induce the endothelial changes described above. Elevated levels of these particular cytokines are responsible for significant capillary immunopathology in many inflammatory disorders. The use of anti-TNFα therapy (e.g., etanercept, infliximab) has proven to be of some value in rheumatoid arthritis and other autoimmune disorders in which TNFα is a key factor in tissue destruction.12 The antiendothelial antibodies common in autoimmune diseases, such as systemic lupus erythematosus, also bind to glycocalyx components and induce vasculitis and thrombosis.16 17 18 These antibodies attach to β-2-glycoprotein-1 (B2GP1), a positively charged common serum protein that normally binds to the negatively charged components of the glycocalyx. This antibody binding to B2GP1 triggers the typical pathogenic endothelial cell reaction.19 20 Unfortunately, many amino acid sequences on bacteria and viruses share epitopes with B2GP1, making it a target due to molecular mimicry.21 22 23 This allows many common infections to cause endothelial cell pathology, vasculitis, and localized inflammation in locations not typically affected in specific immune disorders. Thus, inflammation can occur away from the primary location of infection due to circulating antibodies and other immune factors. Such sensitivity and pathology are seen in organs requiring a tightly regulated vascular barrier, such as the eye (blood-retina barrier), brain (blood-brain barrier), and ear (blood-labyrinth barrier).
Steroid Treatments For Inflammatory Diseases
The therapeutic glucocorticoids (dexamethasone, prednisolone, and prednisone) have numerous functions that help to reduce inflammation. For example, these medications suppress the production of inflammatory cells and cause apoptosis of existing cells that proliferated during inflammation. Another key function of steroids is to suppress the production of inflammatory cytokines by the various immune cells and endothelial cells within the inflammatory site. Glucocorticoids also suppress the production of antibodies against foreign antigens (infections) or the body's own proteins that are sometimes perceived as antigens (in autoimmune disease). Activation of the glucocorticoid receptor also can stimulate the production of inhibitory factors that interrupt various inflammatory cascades within cells.24 Finally, steroids trigger up-regulation of endothelial cell genes involved in the production of junctional proteins to reseal the capillary lining and reduce movement of these factors into the tissues.25 26 27
Inner Ear Vascular Pathophysiology
The high metabolic demands of the inner ear require a fully functional vasculature.1 In particular, the stria vascularis and underlying spiral ligament have unique homeostatic functions that require an uncompromised local blood flow. Endothelial cells of the stria vessels are connected by tight junctions to establish the blood-labyrinth barrier and control the movement of circulating inflammatory cells and other large proteins. This barrier also allows the endolymph to maintain the high potassium (K+) levels required for the endocochlear potential (EP) and normal cochlear function. Any compromise of this barrier function by vascular leakage would lead to an immediate hearing loss. Furthermore, these cochlear lateral wall capillaries are physically connected with other cells via gap junctions for effectively moving K+ through the stria and into the endolymph.28 This critical transport function of lateral wall structures requires a tightly regulated vascular supply and blood-labyrinth barrier. Table 1 summarizes the vascular issues that are potential links between systemic inflammation and inner ear disease.
Table 1. Vascular Factors in Ear Disorders.
| Systemic Vasculature carries hormones responsible for normal organ function. Vasculature carries immune factors from systemic infectious and inflammatory diseases. Endothelial cells are active participants in tissue response to circulating inflammatory factors. Endothelial cell tight junctions are opened for extracapillary movement of serum factors. Inner ear Vasculature is the connection between the body and the ear. Vascular endothelial cells are the gatekeepers to the ear. Nothing enters the ear without passing either through or between endothelial cells. Serum inflammatory factors are commonly seen in numerous hearing disorders. Breakdown of the blood-labyrinth barrier is the first vascular reaction to inflammation. Steroids cause blood-labyrinth barrier restoration by up-regulating tight junction genes. |
Because the cochlear vasculature is very sensitive to circulating inflammatory factors, hearing and vestibular functions can become at risk in even the most minor of vascular changes (Table 1). The normal vascular reaction to inflammatory factors would be harmless in most organs, but in the ear this can lead to breakdown of strial integrity and loss of the blood-labyrinth barrier (Fig. 1), changes that would ultimately be detrimental to endolymph production and maintenance of the EP.29 30 The anionic sites on lateral wall vascular endothelial cells that are stripped off by immune reactions presumably include negatively charged glycocalyx components.31 32 However, because the lateral wall can repair itself, hearing loss that is not due to permanent changes in the sensory organ can be restored. Glucocorticoids, which are known to cause tight junctions to reform,26 27 are often used to treat reversible hearing loss. Studies in autoimmune mice show that circulating inflammatory factors cause suppression of numerous inner ear ion homeostasis genes, including the tight junction and gap junction genes.33 34 However, their gene expression is restored by glucocorticoid therapy.
Vascular Pathophysiology In Hearing Disorders
Systemic inflammatory factors may cause hearing loss by disrupting vascular endothelial cell integrity in the stria, causing breakdown of the blood-labyrinth barrier and endolymph ion homeostasis. This theory was proposed as far back as 1953 by Hilger as a potential explanation for sudden hearing loss.35 Elevated inflammatory cytokines have been measured in various types of hearing loss (Table 2), suggesting the sensitivity of the inner ear to these circulating immune factors. The potential involvement of cytokines in hearing loss may represent a final common pathway for numerous systemic immune-mediated conditions, and some of the changes might be facilitated by genetic components. Ménière's disease and sudden hearing loss have been correlated with altered genes for the cytokine interleukin-1α.36 Also, defects in expression of interleukin-1β and its receptor are correlated with steroid-responsive autoimmune hearing loss.37 38 Targeting the cytokine TNFα with blockers for immune-mediated hearing loss has met with inconsistent results. Positive39 40 and negative41 42 results with such drugs suggest that TNFα may vary in its pathogenic role in different patients.
Table 2. Inflammatory Cytokines in Hearing Disorders.
| Interleukin-1α36 85 86 |
| Interleukin-1β37 85 |
| Interleukin-1 receptor 238 |
| Interleukin-487 88 |
| Interleukin-563 88 |
| Interleukin-689 |
| Interleukin 8 (human/MIP-2 mouse)85 |
| Interleukin-1075 88 |
| Interleukin-1388 |
| Transforming growth factor-β85 88 |
| Tumor necrosis factor-α24 58 75 85 86 90 91 92 |
| Interferon-γ62 63 75 88 93 |
| Vascular endothelial growth factor57 58 94 |
| Matrix metalloproteinase-389 |
Immune-mediated vascular disruption in the stria vascularis could explain a final common pathway for a variety of steroid-responsive hearing disorders. A vascular etiology has been proposed for immune-mediated hearing loss,43 44 45 46 Ménière's disease,47 48 49 50 and sudden hearing loss.51 52 53 54 Even the numerous putative antibody-mediated causes55 56 of hearing loss would seem to employ disruption of endothelial barriers to access the inner ear. Noise, ototoxic drugs, and trauma also cause inflammatory processes within the cochlear vasculature, which places at risk ion transport functions required for endolymph production.1 24 57 58 59 Viral infections have been shown to induce movement of immune cells through vascular endothelial cells.60 Thus, proposed cell-mediated mechanisms for hearing loss also would involve disruption of the barrier to permit inflammatory cells access to cochlear tissues.61 62 63 Hearing loss following viral and bacterial infections also can include the antiendothelial (antiphospholipid) antibody attack of glycocalyx components.22 23 64
Such a vascular theory also fits with what we know about hearing and vestibular dysfunction in systemic autoimmune diseases. All known systemic autoimmune diseases have a very high incidence of inner ear disease, generally running 30 to 50%, and vascular compromise in the ear is the proposed etiology because it is common in such diseases.65 66 67 68 69 These cases often have high levels of antiendothelial and antiphospholipid antibodies,43 70 71 72 73 74 75 which are known to target the glycocalyx. Many cases of autoimmune hearing loss occur as sudden hearing loss.46 70 71 76 77 78 79 In fact, often the inner ear is the first organ affected in systemic autoimmune diseases, probably due to the fact that a mild vascular pathology affects the ear faster than any other organ system. In studies of autoimmune mice, the primary defects in the inner ear include breakdown of the stria vascularis blood vessels,80 loss of blood-labyrinth barrier integrity,81 82 loss of EP,83 and hearing loss,84 all of which are restored by glucocorticoid treatments, such as dexamethasone, prednisone, and prednisolone. These medications may serve two functions: suppressing systemic inflammation that caused the inner ear dysfunction in the first place, and also reestablishing ionic homeostasis within the ear. The therapeutic glucocorticoids have a significant effect on recovery of ion transporters and channels, as well as up-regulating genes for tight junction proteins.33 34 Better characterization of the vascular pathophysiology in the ear, along with increased knowledge regarding which steroids best induce recovery of those cellular and molecular processes at risk, will help guide efforts to develop more targeted treatments in the future.
Summary
Sensitivity of the cochlear vasculature to circulating inflammatory factors places the ear at considerable risk. Endothelial cell activation by circulating inflammatory factors is a natural cellular process by which the body regulates and controls infection. This process is part of the innate immune response and is necessary for survival. However, elements of this process that are virtually inconsequential to other organs can be highly detrimental to the cochlea. These natural inflammatory processes compromise the critical vascular endothelial cell blood-labyrinth barriers of the cochlea and cause hearing loss. This final common pathway from systemic inflammation is likely responsible for a variety of hearing disorders. Because the lateral wall of the cochlea can repair itself and restore normal functions, some spontaneous recovery of hearing is seen in patients with mild hearing loss. As we increase our understanding of the principles of vascular sensitivity and control, we may be able to improve treatments for patients who experience hearing loss due to vascular disruption.
Acknowledgments
This work was supported by Grant NIH-NIDCD R01 DC05593 (D.R.T.).
References
- 1.Shi X. Physiopathology of the cochlear microcirculation. Hear Res. 2011;282:10–24. doi: 10.1016/j.heares.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reitsma S, Slaaf D W, Vink H, van Zandvoort M A, oude Egbrink M G. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor K R, Gallo R L. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 4.Pries A R, Kuebler W M. Normal endothelium. Handb Exp Pharmacol. 2006;(176 Pt 1):1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- 5.Bernfield M, Götte M, Park P W. et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 6.Broekhuizen L N, Mooij H L, Kastelein J J, Stroes E S, Vink H, Nieuwdorp M. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr Opin Lipidol. 2009;20:57–62. doi: 10.1097/MOL.0b013e328321b587. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwdorp M, Meuwese M C, Vink H, Hoekstra J B, Kastelein J J, Stroes E S. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol. 2005;16:507–511. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 8.Aird W C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 9.Woodfin A, Voisin M B, Imhof B A, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Q, McKeown S J, Santos L. et al. Macrophage migration inhibitory factor increases leukocyte-endothelial interactions in human endothelial cells via promotion of expression of adhesion molecules. J Immunol. 2010;185:1238–1247. doi: 10.4049/jimmunol.0904104. [DOI] [PubMed] [Google Scholar]
- 11.Mulivor A W, Lipowsky H H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwdorp M, Meuwese M C, Mooij H L. et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Marcus B C Hynes K L Gewertz B L Loss of endothelial barrier function requires neutrophil adhesion Surgery 1997122420–426.; discussion 426–427 [DOI] [PubMed] [Google Scholar]
- 14.Iversen P O, Nicolaysen A, Kvernebo K, Benestad H B, Nicolaysen G. Human cytokines modulate arterial vascular tone via endothelial receptors. Pflugers Arch. 1999;439:93–100. doi: 10.1007/s004249900149. [DOI] [PubMed] [Google Scholar]
- 15.Henry C B, Duling B R. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 16.Meroni P L, Borghi M O, Raschi E. et al. Inflammatory response and the endothelium. Thromb Res. 2004;114:329–334. doi: 10.1016/j.thromres.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Raschi E, Testoni C, Bosisio D. et al. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 18.Del Papa N, Sheng Y H, Raschi E. et al. Human beta 2-glycoprotein I binds to endothelial cells through a cluster of lysine residues that are critical for anionic phospholipid binding and offers epitopes for anti-beta 2-glycoprotein I antibodies. J Immunol. 1998;160:5572–5578. [PubMed] [Google Scholar]
- 19.Hamid C, Norgate K, D'Cruz D P. et al. Anti-beta2GPI-antibody-induced endothelial cell gene expression profiling reveals induction of novel pro-inflammatory genes potentially involved in primary antiphospholipid syndrome. Ann Rheum Dis. 2007;66:1000–1007. doi: 10.1136/ard.2006.063909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierangeli S S, Chen P P, González E B. Antiphospholipid antibodies and the antiphospholipid syndrome: an update on treatment and pathogenic mechanisms. Curr Opin Hematol. 2006;13:366–375. doi: 10.1097/01.moh.0000239710.47921.d2. [DOI] [PubMed] [Google Scholar]
- 21.Harel M, Aron-Maor A, Sherer Y, Blank M, Shoenfeld Y. The infectious etiology of the antiphospholipid syndrome: links between infection and autoimmunity. Immunobiology. 2005;210:743–747. doi: 10.1016/j.imbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Blank M, Asherson R A, Cervera R, Shoenfeld Y. Antiphospholipid syndrome infectious origin. J Clin Immunol. 2004;24:12–23. doi: 10.1023/B:JOCI.0000018058.28764.ce. [DOI] [PubMed] [Google Scholar]
- 23.Blank M, Barzilai O, Shoenfeld Y. Molecular mimicry and auto-immunity. Clin Rev Allergy Immunol. 2007;32:111–118. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- 24.Abi-Hachem R N, Zine A, Van De Water T R. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Patents CNS Drug Discov. 2010;5:147–163. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]
- 25.Chappell D, Hofmann-Kiefer K, Jacob M. et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 26.Felinski E A, Antonetti D A. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res. 2005;30:949–957. doi: 10.1080/02713680500263598. [DOI] [PubMed] [Google Scholar]
- 27.Underwood J L, Murphy C G, Chen J. et al. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am J Physiol. 1999;277(2 Pt 1):C330–C342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- 28.Dai M, Shi X. Fibro-vascular coupling in the control of cochlear blood flow. PLoS ONE. 2011;6:e20652. doi: 10.1371/journal.pone.0020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagaya M, Yamazaki M, Teranishi M. et al. Endolymphatic hydrops and blood-labyrinth barrier in Ménière's disease. Acta Otolaryngol. 2011;131:474–479. doi: 10.3109/00016489.2010.534114. [DOI] [PubMed] [Google Scholar]
- 30.Scherer E Q, Yang J, Canis M. et al. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41:2618–2624. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomoda K, Yamawaki T, Suzuka Y, Yamashita T, Kumazawa T. Alterations of charge barrier in the inner ear following immune reactions. Ann Otol Rhinol Laryngol Suppl. 1992;157:63–66. doi: 10.1177/0003489492101s1013. [DOI] [PubMed] [Google Scholar]
- 32.Torihara K, Suganuma T, Ide S, Morimitsu T. Anionic sites in blood capillaries of the mouse cochlear duct. Hear Res. 1994;77:69–74. doi: 10.1016/0378-5955(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 33.Trune D R, Hausman F, Kempton B. Baltimore, MD: 2011. Cochlear ion homeostasis mechanisms are suppressed in autoimmune inner ear disease and restored by glucocorticoid treatment. [Google Scholar]
- 34.Trune D R. Ion homeostasis in the ear: mechanisms, maladies, and management. Curr Opin Otolaryngol Head Neck Surg. 2010;18:413–419. doi: 10.1097/MOO.0b013e32833d9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilger J A. The common ground of allergy, autonomic dysfunction and endocrine imbalance. Trans Am Acad Ophthalmol Otolaryngol. 1953;57:443–446. [PubMed] [Google Scholar]
- 36.Furuta T, Teranishi M, Uchida Y. et al. Association of interleukin-1 gene polymorphisms with sudden sensorineural hearing loss and Ménière's disease. Int J Immunogenet. 2011;38:249–254. doi: 10.1111/j.1744-313X.2011.01004.x. [DOI] [PubMed] [Google Scholar]
- 37.Pathak S, Goldofsky E, Vivas E X, Bonagura V R, Vambutas A. IL-1β is overexpressed and aberrantly regulated in corticosteroid nonresponders with autoimmune inner ear disease. J Immunol. 2011;186:1870–1879. doi: 10.4049/jimmunol.1002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vambutas A, DeVoti J, Goldofsky E, Gordon M, Lesser M, Bonagura V. Alternate splicing of interleukin-1 receptor type II (IL1R2) in vitro correlates with clinical glucocorticoid responsiveness in patients with AIED. PLoS ONE. 2009;4:e5293. doi: 10.1371/journal.pone.0005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Wijk F, Staecker H, Keithley E, Lefebvre P P. Local perfusion of the tumor necrosis factor alpha blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiol Neurootol. 2006;11:357–365. doi: 10.1159/000095897. [DOI] [PubMed] [Google Scholar]
- 40.Rahman M U, Poe D S, Choi H K. Etanercept therapy for immune-mediated cochleovestibular disorders: preliminary results in a pilot study. Otol Neurotol. 2001;22:619–624. doi: 10.1097/00129492-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Matteson E L, Choi H K, Poe D S. et al. Etanercept therapy for immune-mediated cochleovestibular disorders: a multi-center, open-label, pilot study. Arthritis Rheum. 2005;53:337–342. doi: 10.1002/art.21179. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S, Shoup A, Weisman M H, Harris J. Etanercept treatment for autoimmune inner ear disease: results of a pilot placebo-controlled study. Otol Neurotol. 2005;26:903–907. doi: 10.1097/01.mao.0000185082.28598.87. [DOI] [PubMed] [Google Scholar]
- 43.George D L, Pradhan S. Idiopathic sensorineural hearing disorders in adults—a pragmatic approach. Nat Rev Rheumatol. 2009;5:505–512. doi: 10.1038/nrrheum.2009.150. [DOI] [PubMed] [Google Scholar]
- 44.Kanzaki J, Kanzaki S, Ogawa K. Long-term prognosis of steroid-dependent sensorineural hearing loss. Audiol Neurootol. 2009;14:26–34. doi: 10.1159/000151587. [DOI] [PubMed] [Google Scholar]
- 45.Dornhoffer J L, Arenberg J G, Arenberg I K, Shambaugh G E Jr. Pathophysiological mechanisms in immune inner ear disease. Acta Otolaryngol Suppl. 1997;526:30–36. doi: 10.3109/00016489709124018. [DOI] [PubMed] [Google Scholar]
- 46.Amor Dorado J C, Barreira Fernández MdelP, Regueiro Villarin S, González-Gay M A. [Audiovestibular manifestations in systemic vasculitis] Acta Otorrinolaringol Esp. 2009;60:432–442. doi: 10.1016/j.otorri.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Brookes G B. Circulating immune complexes in Meniere's disease. Arch Otolaryngol Head Neck Surg. 1986;112:536–540. doi: 10.1001/archotol.1986.03780050060010. [DOI] [PubMed] [Google Scholar]
- 48.Derebery M J, Rao V S, Siglock T J, Linthicum F H, Nelson R A. Menière's disease: an immune complex-mediated illness? Laryngoscope. 1991;101:225–229. doi: 10.1288/00005537-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Kariya S, Cureoglu S, Fukushima H. et al. Vascular findings in the stria vascularis of patients with unilateral or bilateral Ménière's disease: a histopathologic temporal bone study. Otol Neurotol. 2009;30:1006–1012. doi: 10.1097/MAO.0b013e3181b4ec89. [DOI] [PubMed] [Google Scholar]
- 50.Savastano M, Giacomelli L, Marioni G. Non-specific immunological determinations in Meniere's disease: any role in clinical practice? Eur Arch Otorhinolaryngol. 2007;264:15–19. doi: 10.1007/s00405-006-0147-2. [DOI] [PubMed] [Google Scholar]
- 51.Psifidis A D, Psillas G K, Daniilidis JCh. Sudden sensorineural hearing loss: long-term follow-up results. Otolaryngol Head Neck Surg. 2006;134:809–815. doi: 10.1016/j.otohns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Merchant S N Durand M L Adams J C Sudden deafness: is it viral? ORL J Otorhinolaryngol Relat Spec 20087052–60.; discussion 60–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazarini P R, Camargo A C. Idiopathic sudden sensorineural hearing loss: etiopathogenic aspects. Braz J Otorhinolaryngol. 2006;72:554–561. doi: 10.1016/S1808-8694(15)31004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans K L, Baldwin D L, Bainbridge D, Morrison A W. Immune status in patients with Menière's disease. Arch Otorhinolaryngol. 1988;245:287–292. doi: 10.1007/BF00464632. [DOI] [PubMed] [Google Scholar]
- 55.Brookes G B. Immune complex-associated deafness: preliminary communication. J R Soc Med. 1985;78:47–55. doi: 10.1177/014107688507800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agrup C, Luxon L M. Immune-mediated inner-ear disorders in neuro-otology. Curr Opin Neurol. 2006;19:26–32. doi: 10.1097/01.wco.0000194143.02171.46. [DOI] [PubMed] [Google Scholar]
- 57.Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol. 2009;174:1692–1704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou J, Pyykkö I, Sutinen P, Toppila E. Vibration induced hearing loss in guinea pig cochlea: expression of TNF-alpha and VEGF. Hear Res. 2005;202:13–20. doi: 10.1016/j.heares.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Dai M, Wilson T M. et al. Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS ONE. 2011;6:e16547. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stearns G S, Keithley E M, Harris J P. Development of high endothelial venule-like characteristics in the spiral modiolar vein induced by viral labyrinthitis. Laryngoscope. 1993;103:890–898. doi: 10.1288/00005537-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Solares C A, Edling A E, Johnson J M. et al. Murine autoimmune hearing loss mediated by CD4+ T cells specific for inner ear peptides. J Clin Invest. 2004;113:1210–1217. doi: 10.1172/JCI18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenz R R, Solares C A, Williams P. et al. Interferon-gamma production to inner ear antigens by T cells from patients with autoimmune sensorineural hearing loss. J Neuroimmunol. 2002;130:173–178. doi: 10.1016/s0165-5728(02)00190-x. [DOI] [PubMed] [Google Scholar]
- 63.Baek M J, Park H M, Johnson J M. et al. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol. 2006;177:4203–4210. doi: 10.4049/jimmunol.177.6.4203. [DOI] [PubMed] [Google Scholar]
- 64.Lunardi C, Bason C, Leandri M. et al. Autoantibodies to inner ear and endothelial antigens in Cogan's syndrome. Lancet. 2002;360:915–921. doi: 10.1016/S0140-6736(02)11028-2. [DOI] [PubMed] [Google Scholar]
- 65.Roverano S, Cassano G, Paira S. et al. Asymptomatic sensorineural hearing loss in patients with systemic lupus erythematosus. J Clin Rheumatol. 2006;12:217–220. doi: 10.1097/01.rhu.0000242777.71604.69. [DOI] [PubMed] [Google Scholar]
- 66.Nacci A, Dallan I, Monzani F. et al. Elevated antithyroid peroxidase and antinuclear autoantibody titers in Ménière's disease patients: more than a chance association? Audiol Neurootol. 2010;15:1–6. doi: 10.1159/000218357. [DOI] [PubMed] [Google Scholar]
- 67.Mathews J, Kumar B N. Autoimmune sensorineural hearing loss. Clin Otolaryngol Allied Sci. 2003;28:479–488. doi: 10.1046/j.0307-7772.2003.00738.x. [DOI] [PubMed] [Google Scholar]
- 68.Kastanioudakis I, Ziavra N, Voulgari P V, Exarchakos G, Skevas A, Drosos A A. Ear involvement in systemic lupus erythematosus patients: a comparative study. J Laryngol Otol. 2002;116:103–107. doi: 10.1258/0022215021910032. [DOI] [PubMed] [Google Scholar]
- 69.Berrocal J R, Ramírez-Camacho R. Sudden sensorineural hearing loss: supporting the immunologic theory. Ann Otol Rhinol Laryngol. 2002;111:989–997. doi: 10.1177/000348940211101107. [DOI] [PubMed] [Google Scholar]
- 70.Cadoni G, Fetoni A R, Agostino S. et al. Autoimmunity in sudden sensorineural hearing loss: possible role of anti-endothelial cell autoantibodies. Acta Otolaryngol Suppl. 2002;(548):30–33. doi: 10.1080/00016480260094947. [DOI] [PubMed] [Google Scholar]
- 71.Ottaviani F, Cadoni G, Marinelli L. et al. Anti-endothelial autoantibodies in patients with sudden hearing loss. Laryngoscope. 1999;109(7 Pt 1):1084–1087. doi: 10.1097/00005537-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 72.Mouadeb D A, Ruckenstein M J. Antiphospholipid inner ear syndrome. Laryngoscope. 2005;115:879–883. doi: 10.1097/01.MLG.0000158666.15447.37. [DOI] [PubMed] [Google Scholar]
- 73.Yehudai D, Shoenfeld Y, Toubi E. The autoimmune characteristics of progressive or sudden sensorineural hearing loss. Autoimmunity. 2006;39:153–158. doi: 10.1080/08916930500499599. [DOI] [PubMed] [Google Scholar]
- 74.Toubi E, Ben-David J, Kessel A, Halas K, Sabo E, Luntz M. Immune-mediated disorders associated with idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2004;113:445–449. doi: 10.1177/000348940411300605. [DOI] [PubMed] [Google Scholar]
- 75.Hajas A, Szodoray P, Barath S. et al. Sensorineural hearing loss in patients with mixed connective tissue disease: immunological markers and cytokine levels. J Rheumatol. 2009;36:1930–1936. doi: 10.3899/jrheum.081314. [DOI] [PubMed] [Google Scholar]
- 76.Hervier B, Bordure P, Audrain M, Calais C, Masseau A, Hamidou M. Systematic screening for nonspecific autoantibodies in idiopathic sensorineural hearing loss: no association with steroid response. Otol Neurotol. 2010;31:687–690. doi: 10.1097/MAO.0b013e3181dd13cc. [DOI] [PubMed] [Google Scholar]
- 77.García-Berrocal J R, Ramírez-Camacho R, Millán I. et al. Sudden presentation of immune-mediated inner ear disease: characterization and acceptance of a cochleovestibular dysfunction. J Laryngol Otol. 2003;117:775–779. doi: 10.1258/002221503770716188. [DOI] [PubMed] [Google Scholar]
- 78.Deroee A F, Huang T C, Morita N, Hojjati M. Sudden hearing loss as the presenting symptom of systemic sclerosis. Otol Neurotol. 2009;30:277–279. doi: 10.1097/MAO.0b013e31819bda52. [DOI] [PubMed] [Google Scholar]
- 79.Agrup C. Immune-mediated audiovestibular disorders in the paediatric population: a review. Int J Audiol. 2008;47:560–565. doi: 10.1080/14992020802282268. [DOI] [PubMed] [Google Scholar]
- 80.Trune D R, Kempton J B, Kessi M. Aldosterone (mineralocorticoid) equivalent to prednisolone (glucocorticoid) in reversing hearing loss in MRL/MpJ-Fas1pr autoimmune mice. Laryngoscope. 2000;110:1902–1906. doi: 10.1097/00005537-200011000-00025. [DOI] [PubMed] [Google Scholar]
- 81.Trune D R. Cochlear immunoglobulin in the C3H/lpr mouse model for autoimmune hearing loss. Otolaryngol Head Neck Surg. 1997;117:504–508. doi: 10.1016/s0194-5998(97)70022-6. [DOI] [PubMed] [Google Scholar]
- 82.Lin D W, Trune D R. Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol Head Neck Surg. 1997;117:530–534. doi: 10.1016/S0194-59989770026-3. [DOI] [PubMed] [Google Scholar]
- 83.Ruckenstein M J, Milburn M, Hu L. Strial dysfunction in the MRL-Fas mouse. Otolaryngol Head Neck Surg. 1999;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. [DOI] [PubMed] [Google Scholar]
- 84.Trune D R, Kempton J B. Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Fas(lpr) autoimmune mice. Hear Res. 2001;155:9–20. doi: 10.1016/s0378-5955(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 85.Trune D R, Larrain B E, Hausman F A, Kempton J B, MacArthur C J. Simultaneous measurement of multiple ear proteins with multiplex ELISA assays. Hear Res. 2011;275:1–7. doi: 10.1016/j.heares.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams J C. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002;23:316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 87.Keles E, Gödekmerdan A, Kalidağ T. et al. Meniere's disease and allergy: allergens and cytokines. J Laryngol Otol. 2004;118:688–693. doi: 10.1258/0022215042244822. [DOI] [PubMed] [Google Scholar]
- 88.Cai Q, Du X, Zhou B, Cai C, Kermany M H, Yoo T. Induction of tolerance by oral administration of beta-tubulin in an animal model of autoimmune inner ear disease. ORL J Otorhinolaryngol Relat Spec. 2009;71:135–141. doi: 10.1159/000212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takatsu M, Higaki M, Kinoshita H, Mizushima Y, Koizuka I. Ear involvement in patients with rheumatoid arthritis. Otol Neurotol. 2005;26:755–761. doi: 10.1097/01.mao.0000178138.19848.bd. [DOI] [PubMed] [Google Scholar]
- 90.Süslü N, Yilmaz T, Gürsel B. Utility of immunologic parameters in the evaluation of Meniere's disease. Acta Otolaryngol. 2009;129:1160–1165. doi: 10.3109/00016480802631966. [DOI] [PubMed] [Google Scholar]
- 91.Süslü N, Yilmaz T, Gürsel B. Utility of anti-HSP 70, TNF-alpha, ESR, antinuclear antibody, and antiphospholipid antibodies in the diagnosis and treatment of sudden sensorineural hearing loss. Laryngoscope. 2009;119:341–346. doi: 10.1002/lary.20050. [DOI] [PubMed] [Google Scholar]
- 92.Aminpour S, Tinling S P, Brodie H A. Role of tumor necrosis factor-alpha in sensorineural hearing loss after bacterial meningitis. Otol Neurotol. 2005;26:602–609. doi: 10.1097/01.mao.0000178121.28365.0d. [DOI] [PubMed] [Google Scholar]
- 93.Fuse T, Hayashi T, Oota N. et al. Immunological responses in acute low-tone sensorineural hearing loss and Ménière's disease. Acta Otolaryngol. 2003;123:26–31. doi: 10.1080/0036554021000028074. [DOI] [PubMed] [Google Scholar]
- 94.Selivanova O, Heinrich U R, Brieger J, Feltens R, Mann W. Fast alterations of vascular endothelial growth factor (VEGF) expression and that of its receptors (Flt-1, Flk-1 and Neuropilin) in the cochlea of guinea pigs after moderate noise exposure. Eur Arch Otorhinolaryngol. 2007;264:121–128. doi: 10.1007/s00405-006-0154-3. [DOI] [PubMed] [Google Scholar]


