FIG. 1.
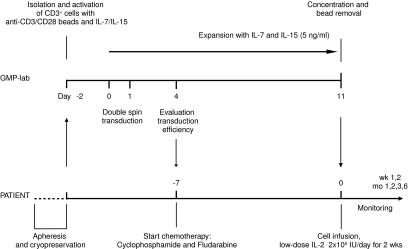
Overview of the GMP T-cell production process and patient conditioning. After thawing of the autologous apheresis product from patients, CD3+ cells are selected and stimulated using anti-CD3/CD28 beads. At days 2 and 3 following activation, cells undergo two cycles of transduction with 1D3HMcys MART-1 TCR pMP71 retroviral supernatant. Subsequently, cells are expanded in the presence of IL-7 and IL-15 (5 ng/ml each) for 11 days. After this expansion phase, cells are concentrated and anti-CD3/CD28 beads are removed. Cells are resuspended in ∼200 ml infusion fluid. Patients will receive a single infusion of autologous, TCR-modified lymphocytes. Before infusion, patients will be treated for 7 days with cyclophosphamide and fludarabine to induce a lymphopenic state. Immediately after cell infusion, patients will be treated with low-dose IL-2 injections subcutaneously for 2 weeks to enhance T-cell survival. GMP, good manufacturing practice.
