Abstract
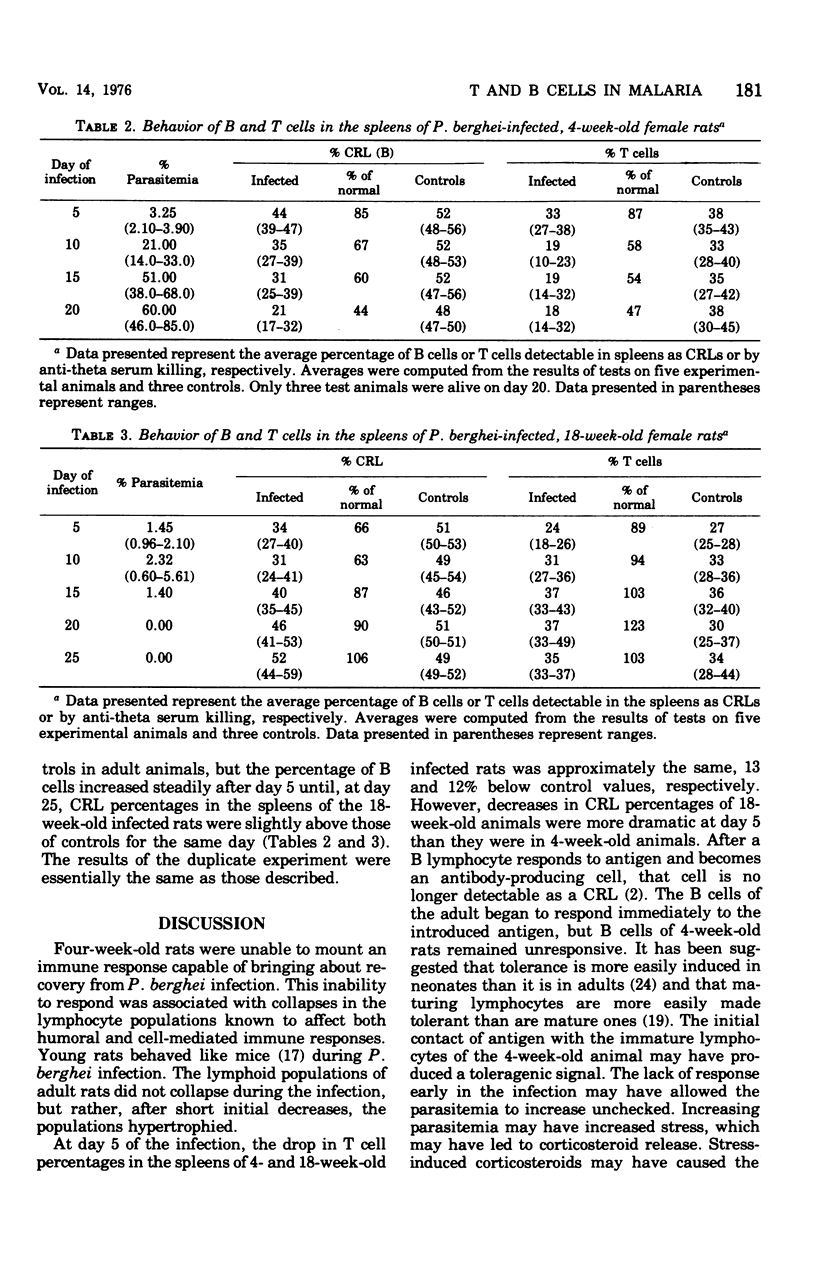
Malaria infection in young rats is characterized by high parasitemia, severe anemia, and death. Parasitemia is lower in older rats, and the rats usually survive. This study was designed to investigate the immunological basis of this difference. T cell numbers in the thymuses and spleens of young (4 weeks old) and in adult (18 weeks old) infected and control rats were determined by killing with anti-theta serum and complement. The number of complement receptor lymphocytes (B cells) in spleens was determined after these cells had formed rosettes with sensitized, complement-coated sheep erythrocytes. Infection in young rats was characterized by progressive and severe thymic involution and by decreasing numbers of T and B cells in the spleen. In 18-week-old rats, T cell numbers in the spleen were slightly below those of controls early in infection but exceeded normal values by day 15. Progressive thymic involution was not a feature of infection in adult rats. The number of complement receptor lymphocytes in the spleens of adult rats decreased dramatically early in infection but were nearly normal by day 15. Severity of malarial infection in young rats is related to the inability of their lymphocytes to respond to Plasmodium berghei antigens early in infection in a way that leads to immunity.
Full text
PDF

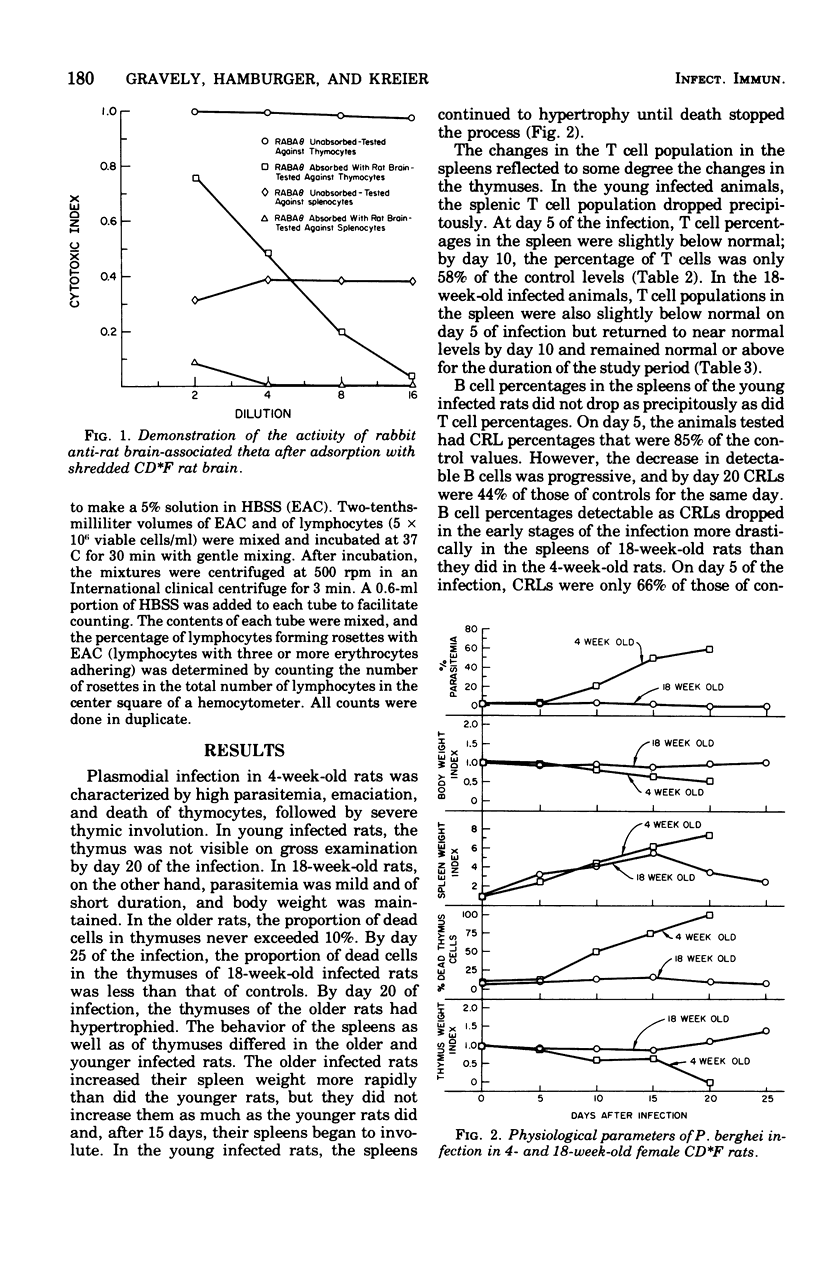


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUCE-CHWATT L. J., GIBSON F. D. Transplacental passage of Plasmodium berghei and passive transfer of immunity in rats and mice. Trans R Soc Trop Med Hyg. 1956 Jan;50(1):47–53. doi: 10.1016/0035-9203(56)90007-4. [DOI] [PubMed] [Google Scholar]
- Barker A. D., Rheins M. S., Pierre R. L. The effect of rabbit anti-mouse brain-associated theta serum on the immunologic responsiveness of AKR mice. Cell Immunol. 1973 Apr;7(1):85–91. doi: 10.1016/0008-8749(73)90184-6. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Allison A. C., Taylor R. B. Plasmodium berghei infections in thymectomized rats. Nature. 1968 Jul 20;219(5151):292–293. doi: 10.1038/219292a0. [DOI] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Properties of protective malarial antibody. Immunology. 1970 Aug;19(2):369–383. [PMC free article] [PubMed] [Google Scholar]
- Douglas T. C. Occurrence of a theta-like antigen in rats. J Exp Med. 1972 Nov 1;136(5):1054–1062. doi: 10.1084/jem.136.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol. 1974 Jul;113(1):127–139. [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Golub E. S. The distribution of brain-associated theta antigen cross-reactive with mouse in the brain of other species. J Immunol. 1972 Jul;109(1):168–170. [PubMed] [Google Scholar]
- Greenwood B. M. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1974 Mar 16;1(7855):435–436. doi: 10.1016/s0140-6736(74)92386-1. [DOI] [PubMed] [Google Scholar]
- Hamburger J., Kreier J. P. Antibody-mediated elimination of malaria parasites (plasmodium berghei) in vivo. Infect Immun. 1975 Aug;12(2):339–345. doi: 10.1128/iai.12.2.339-345.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Kreier J. P. Mechanisms of erythrocyte destruction in chickens infected with Plasmodium gallinaceum. Mil Med. 1969 Sep;134(10):1203–1219. [PubMed] [Google Scholar]
- Krettli A. U., Nussenzweig R. Depletion of T and B lymphocytes during malarial infections. Cell Immunol. 1974 Sep;13(3):440–446. doi: 10.1016/0008-8749(74)90263-9. [DOI] [PubMed] [Google Scholar]
- Moran C. J., De Rivera V. S., Turk J. L. The immunological significance of histological changes in the spleen and liver in mouse malaria. Clin Exp Immunol. 1973 Mar;13(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira D. T., Silverman P. H., Gaines C. Anti-thymocyte serum effects on Plasmodium berghei infection in rats. Immunology. 1970 Nov;19(5):759–766. [PMC free article] [PubMed] [Google Scholar]
- Stechschulte D. J. Plasmodium berghei infection in thymectomized rats. Proc Soc Exp Biol Med. 1969 Jul;131(3):748–752. doi: 10.3181/00379727-131-33968. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN A. Blood loss and replacement in plasmodial infections. II. Plasmodium vinckel in untreated weanling and mature rats. J Infect Dis. 1958 Nov-Dec;103(3):205–224. doi: 10.1093/infdis/103.3.205. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN A., YOELI M. Age and sex as factors influencing Plasmodium berghei infections in intact and splenectomized rats. J Infect Dis. 1954 May-Jun;94(3):225–236. doi: 10.1093/infdis/94.3.225. [DOI] [PubMed] [Google Scholar]


