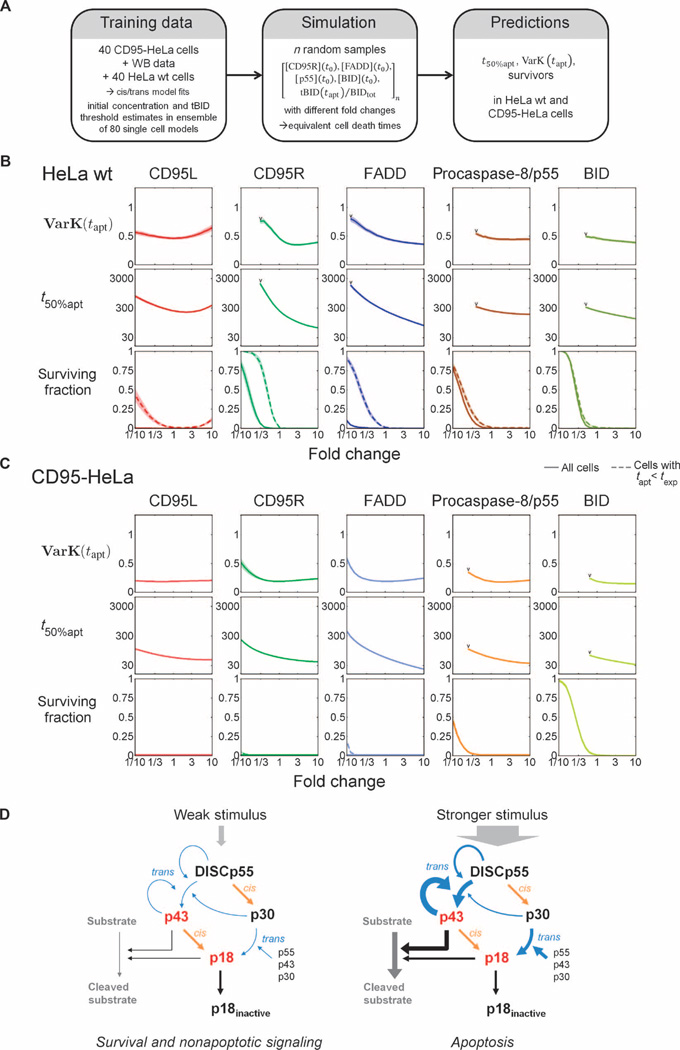
Fig. 7. Determinants of cell death kinetics and variability.
(A) To study how concentrations of signaling proteins along the cell death pathway control the dynamics of apoptosis, we used fits of the cis/trans model to the complete set of CD95-HeLa and HeLa wt cell data to generate log-normal multivariate joint distributions of initial protein concentrations and tBID thresholds for the two cell lines. For simulations, random vectors of initial concentrations and tBID threshold fractions were sampled, and simulations were conducted with different fold changes of initial concentrations. (B and C) Predicted coefficients of variation for cell death times [VarK(tapt)], median cell death times (t50%apt), and fractions of surviving cells at different fold changes of initial protein concentrations for CD95L, CD95R, FADD, p55, and BID for HeLa wt (B) and CD95-HeLa cells (C). Predictions at fold change = 1 represent conditions at [CD95L] = 500 ng/ml. In the subplots for surviving fractions, dashed lines represent the surviving fractions within the duration of the single-cell experiments (13 hours), whereas solid lines represent surviving fractions within the total integration time interval. VarK(tapt) and t50%apt are only calculated in cases for which ≥90% of the cells undergo apoptosis (limits marked by “V”). (D) The cis/trans mechanism restricts apoptotic signaling to strong death receptor stimulation. (Left) A weak cell death stimulus generates low caspase-8 cleavage activity: Low numbers of active DISCs generate low amounts of p43 during weak feedback from p43. In this situation, p55 is processed through the inactive intermediate p30 and only non-apoptotic pathways will be activated. (Right) A stronger stimulus enhances production of the active intermediate p43 by causing stronger feedback, therefore increasing the efficiency of the conversion of procaspase-8 into the products with apoptosis-inducing cleavage activity.