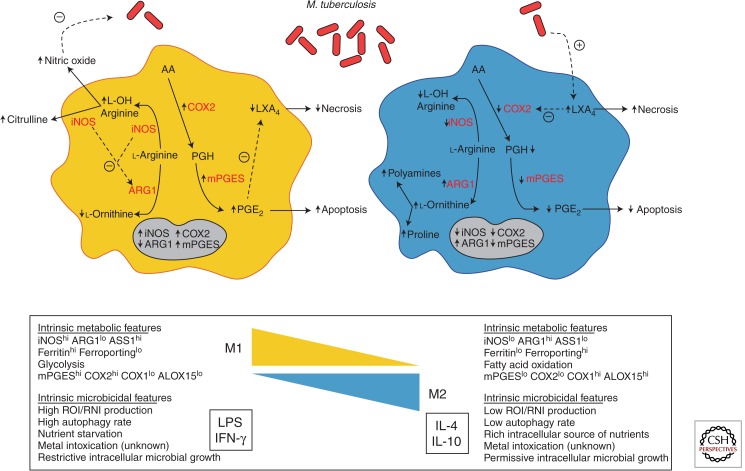
Figure 1.
A model illustrating the modulation of key metabolism pathways via macrophage polarization by M. tuberculosis. On pathogenic challenge, a M1 macrophage (left) shifts its metabolic states in order to support microbicidal activities. Among these, the production of NO (nitric oxide) is an essential effector molecule to control microbial growth. To ensure an optimal NO production, M1 macrophages increase the expression ratio of iNOS (inducible NO synthase) to reduce the expression of ARG1 (arginase 1), and outcompete ARG1 for their common l-arginine substrate. In addition, as the death modality of infected macrophages is an innate immune mechanism, a M1 macrophage shifts the metabolism of arachidonic acid (AA) for the optimal production of PGE2 (Prostaglandin E2) and favors the apoptosis process, which ultimately is thought to lead to the elimination of the pathogen. This is accomplished by the increase of COX2 (prostaglandin-endoperoxide synthase 2) expression that ensures a high ratio of PGE2/LXA4 (lipoxin4). In contrast, a M2 macrophage (right) displays different metabolic states to support different functional programs including tissue maintenance and repair, among others. Indeed, by increasing ARG1 expression and reciprocally diminishing that of iNOS, the metabolism of l-arginine is shifted toward the production of proline and polyamines, which foment collagen production and cell proliferation, respectively. Likewise, M2 macrophages decrease the production PGE2 by arresting the expression of COX2. M. tuberculosis appears to have the capacity to modulate the process of macrophage polarization and influence the metabolic states of infected cells. Upon challenge with M. tuberculosis, the expression of ARG1 is augmented to outcompete the activity of iNOS for l-arginine and, as a consequence, diminish the production of NO. Similarly, M. tuberculosis also affects the AA metabolism by deliberately tilting a low ratio of PGE2/LXA4, driving infected macrophages toward the cell death process of necrosis, escaping intracellular defense mechanisms in a “timely” manner, and disseminating advantageously within the host.