Abstract
Background
Acute coronary syndrome (ACS) patients have a wide spectrum of risks for subsequent cardiovascular events and death. However, there is no simple, convenience scoring system to identify risk of adverse outcomes. We investigated whether CHADS2 and CHA2DS2-VASc scores were useful tools to assess the risk for adverse events among ACS patients.
Methods
This observational prospective study was conducted at 39 hospitals. Totally 3,183 patients with ACS were enrolled, and CHADS2 and CHA2DS2-VASc scores were calculated. The primary endpoint was occurrence of adverse event, including subsequent myocardial infarction, stroke, or death, within 1 year of discharge.
Results
CHADS2 and CHA2DS2-VASc scores were significant predictors of adverse events in separate multivariate regression analyses. A Kaplan-Meier analysis of CHADS2 and CHA2DS2-VASc scores of ≥2 showed a higher rate of adverse events as compared with scores of <2 (P<0.001;log-rank test). CHA2DS2-VASc score was better than CHADS2 score in predicting subsequent adverse events; the area under the receiver operating characteristic curve increased from 0.66 to 0.70 (p<0.001). Patients with CHADS2 scores of 0 or 1 were further classified according to CHA2DS2-VASc score, using a cutoff value of 2. The rate of adverse events significantly differed between those with a score of <2 and those with a score of ≥2 (4.1% vs.10.7%, P<0.001).
Conclusions
CHADS2 and CHA2DS2-VASc scores were useful predictors of subsequent adverse events in ACS patients.
Introduction
Acute coronary syndrome (ACS) is diagnosed when patients present with unstable angina, non-ST-elevation myocardial infarction (MI), or ST-elevation MI. Such patients have a wide spectrum of risks for death and cardiovascular ischemic events.[1]–[3] Careful risk assessment of ACS patients helps clinicians determine prognosis and may therefore be useful in guiding management and providing valuable information to patients. [4], [5] To be clinically practical, a risk stratification model must be straightforward and use clinical risk factors that are readily ascertainable at hospital presentation.
Several scoring methods, including GRACE (Global Registry of Acute Coronary Events) [6], TIMI (Thrombolysis in Myocardial Infarction) [7], and PURSUIT (Platelet glycoprotein IIb/IIIa in Unstable angina: Receptor Suppression Using Integrillin Therapy) [8], are developed in order to distinguish ACS patients at the risk of adverse outcome, who may benefit most from aggressive therapies. However, there is no simple, convenience, and commonly accepted tool for assessing the risk of adverse clinical events such as MI, stroke, or death in patients with ACS. The CHADS2 (congestive heart failure; hypertension; age ≥75 years; type 2 diabetes; and previous stroke, transient ischemic accident [TIA], or thromboembolism [doubled]) score was originally used to estimate the risk of stroke in individuals with atrial fibrillation (AF) but is also a powerful predictor of stroke and death in patients with ischemic heart disease. [9], [10] A high score may be an independent marker of poor prognosis in cardiovascular disease.
The CHA2DS2-VASc score (congestive heart failure; hypertension; age ≥75 years [doubled]; type 2 diabetes; previous stroke, TIA, or thromboembolism [doubled]; vascular disease; age 65–75 years; and sex category) extends the CHADS2 score by considering additional risk factors for stroke and was recently recommended in a guideline for antithrombotic therapy in patients with AF or atrial flutter.[11]–[13] A previous study found that CHADS2 score could identify ACS patients at higher risk of adverse events and that the CHA2DS2-VASc and CHADS2 scores did not significantly differ in their power to predict mortality in ACS patients. [14] However, as compared with CHADS2 score, each additional component of the CHA2DS2-VASc score, such as peripheral vascular disease, female sex, and age 65–74 years, was associated with worse clinical outcomes in ACS patients. As compared with CHADS2 score, the CHA2DS2-VASc score is believed to have better prognostic predictive value for clinical outcomes. However, no published studies have investigated the association of CHADS2 and CHA2DS2-VASc scores with adverse event in patients with ACS. We compared the performance of CHADS2 and CHA2DS2-VASc scores in predicting subsequent MI, stroke, and death in patients with ACS.
Methods
Study design
In this prospective, nationwide, multicenter, non-interventional observational study, each participating site recruited 50–200 consecutive eligible patients. To ensure that the sample satisfactorily represented the ACS population, sites were selected by using data from the Scientific Committee of Taiwan Society of Cardiology. The accuracy of documentation was examined in 5% of case report forms at each recruiting site. Patient data collected included baseline characteristics such as risk factors for cardiovascular disease, clinical presentation, and in-hospital interventions, as well as medications prescribed and clinical outcomes. Participants were followed up at 3, 6, 9, and 12 months after discharge, and the data collected included medication use and clinical adverse events, including MI, stroke, and death.
Patient recruitment
Patients were aged 20 years or older and were admitted to hospital within 24hours of presenting with symptoms of ACS. All patients who provided informed consent were eligible to be included in the study. Patients were excluded from this study if ACS was precipitated by comorbidity, such as trauma, if they were previously enrolled in this trial, or if they were participating in an investigational drug study.
This study was performed in accordance with the Declaration of Helsinki and good clinical practice. Ethics committee approval was obtained at all trial sites including China University Medical Hospital, Taoyuan General Hospital, Wan-Fang Hospital, Show Chwan Memorial Hospital, Chia-Yi Christian Hospital, Kuang Tien General Hospital, National Taiwan University Hospital, Cheng Ching Hospital, Sin Lau Hospital The Presbyterian Church of Taiwan, Tainan Municipal Hospital, Mackay Memorial Hospital, E-Da Hospital, Chi-Mei Hospital, Taichung Armed Forces General Hospital, Taipei Tzu Chi General Hospital, Kaohsiung Medical University Chung-Ho Memorial Hospital, Taichung Veterans General Hospital, Pingtung Christian Hospital, Lo-Tung Po-Ai Hospital, Far Eastern Memorial Hospital, National Cheng Kung University Hospital, National Taiwan University Hospital, Yun-lin Branch, Dalin Tzuchi General Hospital, Kee-lung Hospital, Taipei Veterans General Hospital, Cathay General Hospital, Kaohsiung Veterans General Hospital, Taipei Medical University Hospital, Shin Kong Wu Ho-Su Memorial Hospital, Changhua Christian Hospital, National Taiwan University Hospital, Chung Shan Medical University Hospita, Hualien Tzu Chi General Hospital, Mackay Memorial Hospital, Taitung Branch, Linkou Chang Gung Memorial Hospital, Hsin Chu General Hospital, Kaohsiung Chang Gung Memorial Hospital, Tri-Service General Hospital and Cheng-Hsin Hospital. Written informed consent was obtained from each patient.
Definition of ACS
ACS was defined as a heterogeneous range of symptoms, from ST-elevation MI to unstable angina and non-ST-elevation MI, as previously described. [15] Briefly, ST-elevation MI was defined as presentation with acute chest pain, or overwhelming shortness of breath, together with persistent electrocardiographic ST elevation >1 mm in 2 or more contiguous leads, or with a new or presumed new left-bundle branch block pattern, on electrocardiography. Presentation with acute chest pain, or overwhelming shortness of breath, with no ST elevation but with classical rise and fall of at least one cardiac enzyme (troponin or MB fraction of creatine kinase) was defined as non-ST-elevation MI. Presentation with acute chest pain, or overwhelming shortness of breath, with neither ST elevation nor abnormal cardiac enzymes was defined as unstable angina.
CHADS2, CHA2DS2-VASc and GRACE scores
CHADS2 score was calculated for all patients by assigning 1 point each for the criteria age ≥75 years, hypertension, diabetes mellitus, and heart failure and 2 points for the criterion previous stroke or TIA. For the CHA2DS2-VASc, 2 points were assigned for history of stroke/TIA or thromboembolism and age ≥75 years and 1 point each was assigned for the criteria age 65–75 years, history of hypertension, diabetes mellitus, heart failure, female sex, and vascular disease (defined as prior MI, complex aortic plaque, carotid disease, and peripheral artery disease, including intermittent claudication, previous surgery or percutaneous intervention for the abdominal aorta or vessels of the lower extremities, and arterial and venous thrombosis). [11], [12] The cutoff values used for grouping CHADS2 and CHA2DS2-VASc scores were determined according to values used in earlier studies of the risk of stroke and atrial properties. [12], [16], [17] Besides, the GRACE risk score [6] (age, Killip class, heart rate, systolic BP, ST-segment deviation and cardiac arrest at admission, elevated biomarkers of myocardial necrosis, and baseline creatinine level) were also calculated from data collected at admission.
Statistical analyses
Sample size for the Taiwan ACS full-spectrum registry was calculated as follows. There are about 50,000 new ACS cases per year in Taiwan. On the basis of the known background incidence rate of 0.0025, a sample of 2,395 patients would achieve 80% power to detect an additional incidence rate of 0.003, with a precision of 0.2% and a 95% confidence interval (CI). Assuming a dropout rate of 20%, a sample of 3,000 was considered adequately representative.
Parameters were summarized using mean, median, standard deviation, and interquartile range, where appropriate, for continuous data, and counts or percentages for categorical data. For comparability between groups, the chi-squared test was used for categorical variables, and analysis of variance (ANOVA) was used for continuous variables. Univariate associations of variables with adverse events, including subsequent MI, stroke, and death, were assessed with multivariate logistic regression. For each variable, the hazard ratio (HR), 95% CI, and P value are provided. The cumulative adverse events curves were constructed according to the Kaplan-Meier method. All statistical tests were two-sided, and a p value of <0.05 was considered to indicate statistical significance. Analyses were done using a time to first event approach, without double counting of events in analyses involving composite endpoints. Patients lost to follow-up were censored at the time of last contact, and their vital status was classified as alive and event-free at that time. We assessed the predictive accuracy of CHADS2 and CHA2DS2-VASC scores by using the receiver operating characteristic (ROC) curve analysis. The areas under the ROC (AUCs) for these 2 indices were compared by using De Long’s method. Statistical analysis was performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics of participants and predictors of acute coronary syndrome
During the period from October 2008 through January 2010, 3,183 eligible patients were enrolled at 39 hospitals in Taiwan. The study population had a mean age of 64 years (range, 20–101 years) and comprised 2,483 (78%) men and 700 (22%) women. Of these 3,183 patients, 2,016 (63%) had hypertension, 1,138 (36%) had diabetes mellitus, 1,235 (39%) had hyperlipidemia, 172 (5%) had a history of congestive heart failure, and 287 (9%) had a history of stroke or TIA. In addition, 367 (12%) patients had vascular disease, including 315 with a history of MI and 71 with peripheral vascular disease.
Table 1 shows the baseline clinical characteristics of the patients, stratified using a cutoff value of 2 on the CHADS2 and CHA2DS2-VASC indices. The burden of previous cardiovascular disease was somewhat greater in patients with a CHADS2 or CHA2DS2-VASC score of ≥2. Hypertension was the most important risk factor among patients with a CHADS2 or CHA2DS2-VASC score of ≥2. CHADS2 and CHA2DS2-VASC scores were inversely associated with ST-elevation MI; however, non-ST-elevation MI and unstable angina were more frequent among those with a CHADS2 or CHA2DS2-VASC score of ≥2. CHADS2 and CHA2DS2-VASC scores were inversely associated with primary percutaneous coronary intervention and the level of the MB fraction of creatine kinase. Participants with higher CHADS2 and CHA2DS2-VASC scores were more likely to present with a high Killip class and greater LV systolic dysfunction. There was no significant difference in drug regimen at discharge (including use of dual antiplatelet therapy, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, β-blockers, and statins) between patients with a CHADS2 or CHA2DS2-VASC score of <2 and those with higher scores.
Table 1. Baseline characteristics of patients stratified using a cutoff value of 2 for CHADS2 and CHA2DS2-VASc scores.
| Variable | CHADS2 score | CHA2DS2-VASc score | ||||
| <2 (n = 1,805) | ≥2 (n = 1,378) | P value | <2 (n = 1,242) | ≥2 (n = 1,941) | P value | |
| Age, years | 58.3±12.1 | 71.5±12.1 | <0.001 | 53.4±9.4 | 70.9±11.6 | <0.001 |
| Age 65–75 years | 439 (24.3) | 313 (22.7) | 0.16 | 122 (9.8) | 630 (32.5) | <0.001 |
| Age ≥75 years | 129 (7.1) | 679 (49.3) | <0.001 | 0 (0) | 808 (41.6) | <0.001 |
| Male | 1,546 (85.7) | 937 (68.0) | <0.001 | 1,196 (96.3) | 1,287 (66.3) | <0.001 |
| Medical History | ||||||
| Current smoker | 946 (52.4) | 367 (26.6) | <0.001 | 774 (62.8) | 539 (27.6) | <0.001 |
| Hypertension | 734 (41.2) | 1,282 (93.5) | <0.001 | 421 (33.9) | 1,595 (82.2) | <0.001 |
| Diabetes | 208 (11.6) | 930 (67.7) | <0.001 | 122 (9.8) | 1016 (52.3) | <0.001 |
| Hyperlipidemia | 599 (33.6) | 636 (46.4) | <0.001 | 402 (32.8) | 833 (42.9) | <0.001 |
| Congestive heart failure | 12 (0.7) | 160 (11.6) | <0.001 | 3 (0.2) | 169 (8.7) | <0.001 |
| Previous CAD | 275 (15.2) | 507 (36.8) | <0.001 | 115 (9.3) | 667 (34.3) | <0.001 |
| Previous myocardial infarction | 107 (21.3) | 208 (31.9) | <0.001 | 25 (2.0) | 290 (14.9) | <0.001 |
| Previous stroke/TIA | 0 (0) | 287 (20.8) | <0.001 | 0 (0) | 287 (14.8) | <0.001 |
| Peripheral arterial disease | 13 (0.7) | 58 (4.2) | <0.001 | 2 (0.2) | 69 (3.6) | <0.001 |
| Vascular disease§ | 119 (6.6) | 259 (18.8) | <0.001 | 27 (2.2) | 340 (17.5) | <0.001 |
| History of atrial fibrillation | 30 (1.7) | 73 (5.3) | <0.001 | 8 (0.6) | 95 (4.9) | <0.001 |
| Chronic kidney disease | 314 (17.4) | 609 (44.2) | <0.001 | 171 (13.8) | 752 (38.7) | <0.001 |
| COPD | 40 (2.2) | 83 (6.0) | <0.001 | 14 (1.1) | 109 (5.6) | <0.001 |
| Clinical presentation | ||||||
| ST-elevation MI | 1,120 (62.0) | 583 (42.3) | <0.001 | 822 (66.2) | 881 (45.4) | <0.001 |
| Non-ST elevation MI | 361 (20.0) | 489 (35.5) | <0.001 | 222 (17.9) | 628 (32.4) | <0.001 |
| Unstable angina | 324 (18.0) | 306 (22.2) | <0.001 | 198 (15.9) | 432 (22.3) | <0.001 |
| Killip class ≥III at admission | 238 (13.2) | 289 (21.0) | <0.001 | 135 (10.9) | 392 (20.2) | <0.001 |
| CK-MB maximum, median ug/L | 76.8±131.3 | 46.6±81.3 | <0.001 | 83.2±138.2 | 51.5±92.5 | <0.001 |
| LVSD (LVEF<40%) | 171 (9.5) | 220 (16.0) | <0.001 | 102 (8.2) | 289 (14.9) | <0.001 |
| Procedures | ||||||
| Fibrinolysis therapy | 33 (2.5) | 22 (2.6) | 0.89 | 22 (2.3) | 33 (2.7) | 0.68 |
| PCI | 1,588 (88.1) | 1,092 (79.5) | <0.001 | 1,111 (89.6) | 1,569 (81.0) | <0.001 |
| Primary PCI | 1,016 (56.3) | 490 (35.6) | <0.001 | 725 (58.4) | 781 (40.2) | <0.001 |
| Rescue PCI | 29 (2.1) | 12 (1.4) | 0.26 | 21 (2.2) | 20 (1.6) | 0.34 |
| CABG | 49 (2.7) | 57 (4.1) | 0.03 | 26 (2.1) | 80 (4.1) | 0.002 |
| Medication at discharge | ||||||
| Dual antiplatelet therapy | 1,351 (74.8) | 1,034 (75.0) | 0.93 | 923 (74.3) | 1,462 (75.3) | 0.53 |
| ACEi/ARB | 1,157 (64.1) | 848 (61.5) | 0.07 | 769 (61.9) | 1,236 (63.9) | 0.32 |
| β-blockers | 988 (54.7) | 712 (51.7) | 0.05 | 653 (52.6) | 1,047 (53.9) | 0.45 |
| Statin therapy | 1,091 (60.4) | 833 (60.4) | 1.00 | 745 (60.0) | 1,179 (60.7) | 0.67 |
| VKA | 34 (1.9) | 33 (2.4) | 0.89 | 26 (2.1) | 41 (2.1) | 0.69 |
Values are presented as number (%) or mean ± SD.
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CK-MB, MB fraction of creatine kinase; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; LVSD, left ventricle systolic dysfunction; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Vascular disease defined as previous myocardial infarction or peripheral arterial obstructive disease.
CHADS2 and CHA2DS2-VASc scores and prediction of subsequent MI, stroke, and death
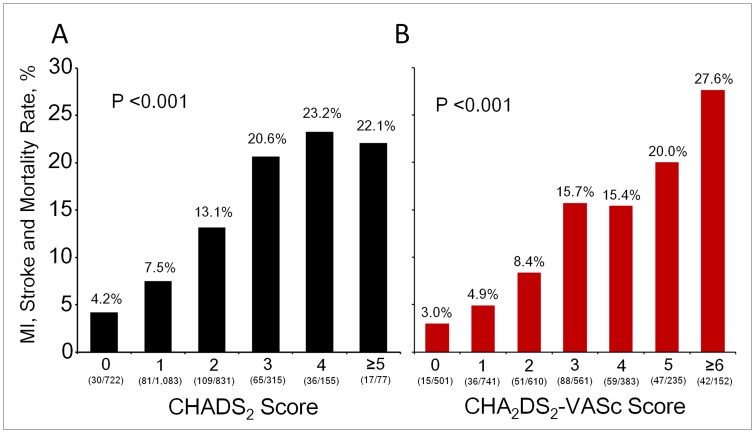
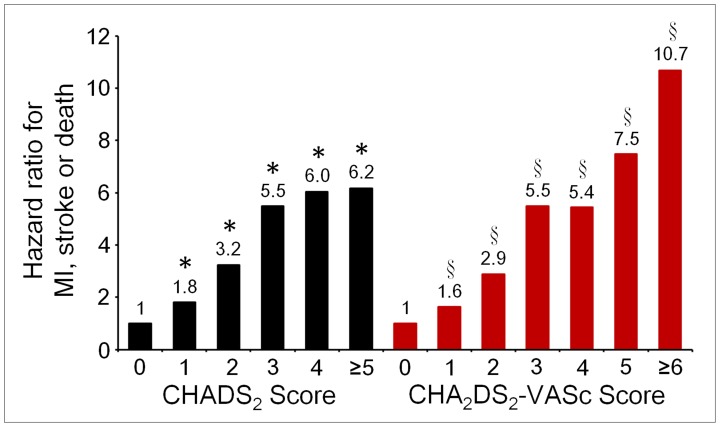
Rates of MI, stroke, and death increased with increasing CHADS2 and CHA2DS2-VASc scores (Fig. 1). Figure 2 shows the HRs for adverse events in relation to CHADS2 and CHA2DS2-VASc scores in patients with ACS. The risk of adverse events progressively increased as CHADS2 and CHA2DS2-VASc scores increased. Clinical outcomes during follow-up, in relation to CHADS2 and CHA2DS2-VASC scores at the cutoff value of 2, are summarized in Table 2. Patients with CHADS2 or CHA2DS2-VASC scores of >2 had higher risks of stroke and death. Overall, a CHADS2 or CHA2DS2-VASC score of >2 was associated with higher risks of MI, stroke, and death during follow-up.
Figure 1. Rates of adverse events, including myocardial infarction (MI), stroke, or death, according to CHADS2 and CHA2DS2-VASc scores.
The rate of MI, stroke, or death increased as CHADS2 (A) and CHA2DS2-VASc (B) scores increased.
Figure 2. Adjusted hazard ratios for the composite endpoint myocardial infarction (MI), stroke, or death, in relation to CHADS2 or CHA2DS2-VASc scores, in patients with acute coronary syndrome.
The risk of MI, stroke, or death progressively increased with each unit increase in CHADS2 and CHA2DS2-VASc scores. The reference groups are patients with scores of 0. * And § are defined as p<0.001 vs. CHADS2 and CHA2DS2-VASc scores of 0, respectively.
Table 2. Clinical outcomes during follow-up stratified using a cutoff value of 2 for CHADS2 and CHA2DS2-VASc scores.
| Variable | CHADS2 score | CHA2DS2-VASc score | ||||
| <2 (n = 1,805) | ≥2 (n = 1,378) | P value | <2 (n = 1,242) | ≥2 (n = 1,941) | P value | |
| Myocardial infarction or stroke | 61 (3.4) | 89 (6.5) | <0.001 | 40 (3.2) | 110 (5.7) | 0.001 |
| Myocardial infarction | 49 (2.7) | 52 (3.8) | 0.09 | 32 (2.6) | 69 (3.6) | 0.13 |
| Stroke | 12 (0.7) | 39 (2.8) | <0.001 | 8 (0.6) | 43 (2.2) | <0.001 |
| Death | 52 (2.9) | 159 (11.5) | <0.001 | 12 (1.0) | 199 (10.3) | <0.001 |
| Myocardial infarction, stroke, or death | 111 (6.2) | 227 (16.5) | <0.001 | 51 (4.1) | 287 (14.8) | <0.001 |
Values are presented as number (%) or mean ± SD.
At a cutoff value of 2, a higher CHADS2 or CHA2DS2-VASC score was significantly associated with rates of MI, stroke or death, before and after adjustment for potential confounders (Table 3). The risk of subsequent MI, stroke, or death increased with every unit increase in CHADS2 or CHA2DS2-VASC score. After adjustment, the HR for future MI, stroke, or death per unit increase in CHADS2 and CHA2DS2-VASC scores was 1.44 (95% CI 1.30–1.58, p<0.001) and 1.36 (95% CI 1.26–1.46, p<0.001), respectively.
Table 3. Hazard ratios for myocardial infarction, stroke, or death according to baseline CHADS2 and CHA2DS2-VASc scores.
| Characteristic | Unadjusted, HR (95% CI) | P Value | Adjusted, HR (95% CI)§ | P Value |
| Myocardial infarction or stroke | ||||
| CHADS2≥2 vs <2 | 2.03 (1.46–2.81) | <0.001 | 1.87 (1.28–2.72) | 0.001 |
| CHADS2 score† | 1.28 (1.13–1.44) | <0.001 | 1.25 (1.08–1.44) | 0.002 |
| CHA2DS2-VASc ≥2 vs <2 | 1.72 (1.19–2.48) | 0.004 | 1.63 (1.10–2.47) | 0.02 |
| CHA2DS2-VASc score† | 1.18 (1.08–1.29) | <0.001 | 1.18 (1.06–1.31) | 0.002 |
| Death | ||||
| CHADS2≥2 vs <2 | 4.40 (3.12–6.06) | <0.001 | 3.17 (2.24–4.47) | <0.001 |
| CHADS2 score† | 1.74 (1.58–1.92) | <0.001 | 1.60 (1.41–1.80) | <0.001 |
| CHA2DS2-VASc ≥2 vs <2 | 11.5 (6.42–20.8) | <0.001 | 8.52 (4.48–16.2) | <0.001 |
| CHA2DS2-VASc score† | 1.62 (1.50–1.75) | <0.001 | 1.55 (1.42–1.70) | <0.001 |
| Myocardial infarction, stroke or death | ||||
| CHADS2≥2 vs <2 | 2.74 (2.19–3.41) | <0.001 | 2.33 (1.81–2.99) | <0.001 |
| CHADS2 score† | 1.52 (1.40–1.65) | <0.001 | 1.44 (1.30–1.58) | <0.001 |
| CHA2DS2-VASc ≥2 vs <2 | 3.42 (2.58–4.53) | <0.001 | 2.98 (2.17–4.07) | <0.001 |
| CHA2DS2-VASc score† | 1.41 (1.33–1.50) | <0.001 | 1.36 (1.26–1.46) | <0.001 |
Adjusted for all clinical variables in Table 1 (except the 5 or 7 variables included in the CHADS2 and CHA2DS2–VASc scoring systems, respectively), LVEF, Killip class, chronic kidney disease and medication at discharge.
Per unit increase in the original 6- or 8-criteria CHADS2 and CHA2DS2-VASc scoring systems, respectively.
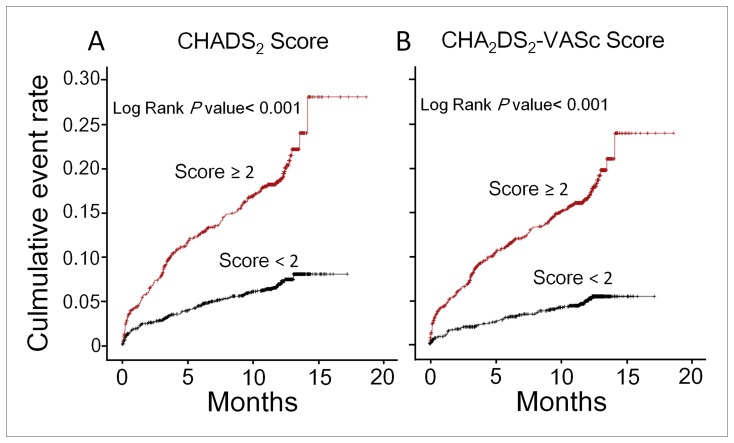
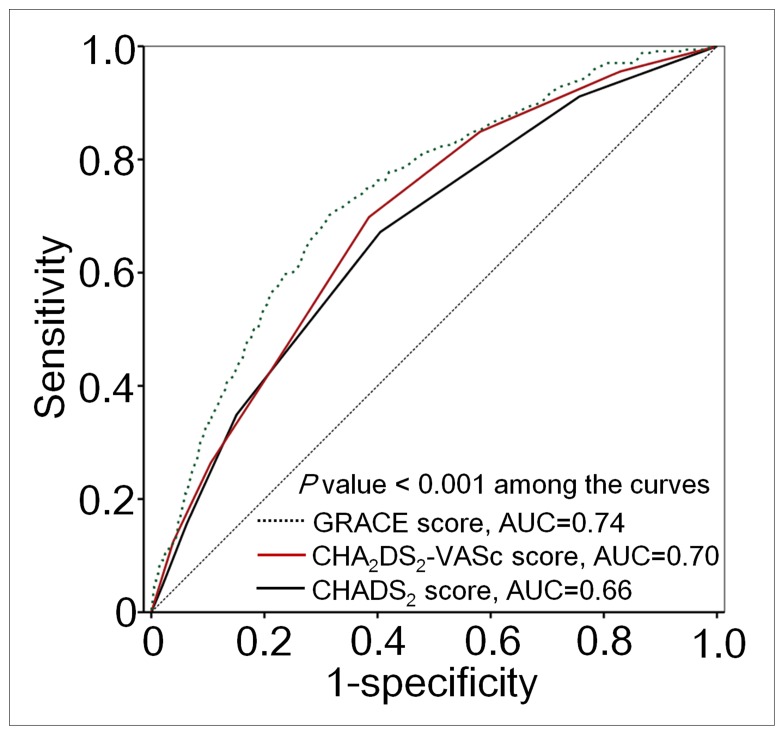
Kaplan-Meier survival analysis revealed that patients with a CHADS2 score of ≥2 had a higher rate of MI, stroke, or death than did those with lower CHADS2 scores (P<0.001, log-rank test; Fig. 3A). Furthermore, a CHA2DS2-VASc score of ≥2 was also a significant predictor of an adverse event (P<0.001, log-rank test; Fig. 3B). However, CHA2DS2-VASc score had better diagnostic performance in predicting the composite endpoint subsequent MI, stroke, or death, as compared with CHADS2 score. The AUC increased from 0.66 to 0.70, and the difference was statistically significant (p<0.001), as shown in Figure 4. The GRACE risk score (AUC = 0.74) had better diagnostic accuracy in predicting adverse events compared with CHA2DS2-VASc score. (p<0.001).
Figure 3. Kaplan-Meier curves for the time to the composite endpoint of myocardial infarction (MI), stroke, or death, according to CHADS2 and CHA2DS2-VASc scores.
Survival analysis showed that a CHADS2 score of ≥2 was associated with a higher event rate than a score of <2 (p<0.001; log-rank test) (A). In addition, a CHA2DS2-VASc score of ≥2 was a significant predictor of adverse events (p<0.001; log-rank test) (B).
Figure 4. Receiver operating characteristic (ROC) curves for CHADS2, CHA2DS2-VASc and GRACE scores predicting myocardial infarction (MI), stroke, or death.
Diagnostic performance in predicting MI, stroke, or death was better for CHA2DS2-VASc score than for CHADS2 score. The area under the ROC curve (AUC) increased from 0.66 to 0.70, and the difference was statistically significant (p<0.001). Besides, the diagnostic accuracy in predicting adverse events was better for GRACE score than for CHA2DS2-VASc score (AUC 0.74 vs. 0.70, p<0.001).
CHA2DS2-VASc score and subsequent adverse events in patients with a CHADS2 score of 0 or 1
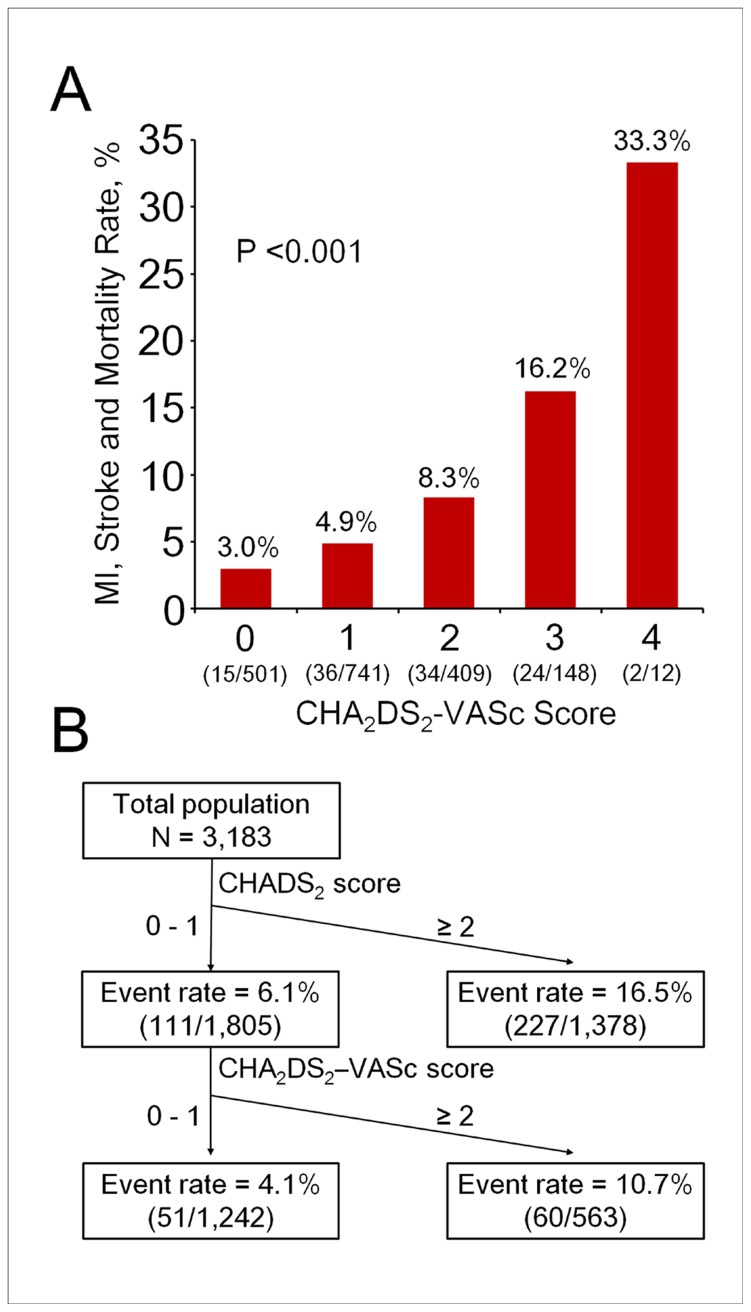
A subgroup analysis of the 1805 patients with CHADS2 scores of 0 or 1 revealed that 111 (6%) had a subsequent MI, stroke, or death, and the rate progressively increased from 3.0% (in patients with CHA2DS2-VASc scores of 0) to 33.3% (in patients with CHA2DS2-VASc scores of 4) (p<0.001; Fig. 5A). Using a CHA2DS2-VASc score of 2 as the cutoff point, patients with a score of ≥2 had a higher event rate than did those with a CHA2DS2-VASc score of <2 (10.7% vs. 4.1%, p<0.001; Fig. 5B).
Figure 5. Flowchart of adverse event rates and risk scores in the patients with CHADS2 score of 0 or 1.
(A) Rate of MI, stroke, or death in patients with a CHADS2 score of 0 or 1, according to CHA2DS2-VASc score. The rate of myocardial infarction (MI), stroke, or death progressively increased, from 3.0% to 33.3%, with increasing CHA2DS2-VASc score. (B) The flowchart shows the rate of MI, stroke, or death in patients stratified by CHADS2 and CHA2DS2-VASc scores.
Discussion
Principal findings
In this study of a cohort of patients with ACS, CHADS2 and CHA2DS2-VASc scores were helpful and convenient indices for predicting subsequent MI, stroke, or death. CHAD2DS2-VASc score was useful for further risk stratification for clinical outcome among patients with CHADS2 scores of 0 or 1. The usefulness of this simple and popular scoring system allows clinicians to summarize the overall risk of MI, stroke, and death in patients with ACS.
CHADS2 score in patients with ACS
The CHADS2 score is a risk index for predicting stroke in patients with AF and can be used to guide anticoagulation therapy. [18], [19] A previous study found that CHADS2 score predicted clinical outcomes in ACS patients with and without AF. [14] It is reasonable to assume that CHADS2 score is valuable in ACS, since each of its components is a prognostic risk factor for ischemic heart disease [20], [21] and stroke. [22], [23] Furthermore, a previous study reported that heart failure, hypertension history, increasing age, and diabetes were independent risk factors for long-term mortality in patients with acute MI. [20] This agrees with our finding that 7% of the present ACS patients with a CHADS2 score of <2, vs. 17% with a score of ≥2, had subsequent MI, stroke, or death. Using multivariate models, we found that CHADS2 score was a powerful predictor of subsequent adverse events after ACS. These findings extend the usefulness of the CHADS2 score in predicting clinical outcomes in patients with ACS. The CHADS2 score may help identify treatable underlying conditions in patients with ACS, thereby decreasing subsequent risk.
CHA2DS2-VASc scores in patients with ACS
The new CHA2DS2-VASc score extends the CHADS2 score by adding the criteria age 65–74 years, vascular disease, and female sex, which increases the predictive value of the CHADS2 for thromboembolic events with low event rates in low-risk patients. [11], [12] A previous study found that CHADS2 and CHA2D2-VASc scores did not significantly differ in relation to prediction of mortality in ACS patients; however, it is important to note that these scoring systems were developed to predict stroke and thromboembolism, not mortality. [14] We found that, as compared with CHADS2 score, CHA2DS2-VASc score had better diagnostic performance in predicting subsequent adverse events. In addition, the AUC significantly increased, from 0.66 to 0.70. Moreover, CHA2DS2-VASc score could further predict risk of subsequent MI, stroke, or death in ACS patients with CHADS2 scores of 0 and 1.
The impact of female sex on ACS has been investigated: as compared with men, women had more complications during hospitalization and a higher mortality rate.[24]–[26] Women and men with ACS had a different clinical outcome, which reflects pathophysiologic and anatomic differences between sexes. [26] Peripheral vascular disease is often complicated by ischemic episodes, not only in peripheral circulation but also in coronary and cerebral vessels. [27], [28] The rate of cardiovascular mortality among patients with peripheral vascular disease was three-fold that of age-matched controls. [29], [30] Furthermore, the presence of peripheral vascular disease in conjunction with ACS is associated with substantial mortality and morbidity. [31] Given that age does not have a binary effect on the risk of adverse events and that age ≥75 years was associated with high risk, it is understandable that the criterion age 65–74 years, in combination with another risk factor, was associated with increased risk in ACS patients.23 It was estimated that 60% of ACS cases were people aged ≥65 years and that 30% were people aged ≥75 years. In addition, as many as 80% of deaths related to ACS occur in patients aged ≥65 years. [32], [33] Taken together, these findings suggest that the risk of subsequent MI, stroke, or death increases with the combination of these additional risk factors in the CHA2DS2-VASc score.
The more complicated GRACE score provided a better prediction for subsequent adverse events than the simpler CHA2DS2-VASc score according to the ROC curve analysis. However, one great advantage of the CHA2DS2-VASc score is that it provides a comprehensive, convenience, and fast method for clinical physician in risk evaluation. No calculators or computers are needed for the risk stratification.
Clinical implications
The CHADS2 scoring system was a simple tool for predicting adverse events among ACS patients. A CHADS2 score of ≥2 was associated with a 16.5% risk of adverse events in ACS patients. Moreover, the more detailed CHA2DS2-VASc scoring system could further discriminate the risk of developing adverse events among patients with a CHADS2 score of 0 or 1. The clinical utility of the CHA2DS2-VASc score should be emphasized, as it was generally believed that patients with a CHADS2 score of 0 or 1 were at low risk; however, among this subgroup, those with a CHA2DS2-VASc score of 4 have a rate of adverse events as high as 33.3%. These findings suggest that CHA2DS2-VASc score is useful in identifying ostensibly low-risk patients who are at risk of adverse events and optimizing management of such patients so as to lower such risk. However, this requires confirmation in a large-scale prospective trial.
Study limitations
This study had several limitations. Patients at other sites might have risk profiles and subsequent outcomes that vary depending on differences in ACS treatment. In addition, adverse events among the present participants would have been missed if such episodes occurred at other hospitals. The incidences of adverse events in the present study may have been underestimated, which would have biased the results against a significant association of CHADS2 and CHA2DS2-VASc scores with adverse events in the present study.
Conclusion
CHADS2 and CHA2DS2-VASc scores can be used to estimate the risk of clinical adverse events in patients with ACS. Among patients with CHADS2 scores of 0 or 1, the CHA2DS2-VASc score was helpful in identifying patients who were at higher risk. These scoring systems could lead to optimization of therapy, which might reduce risks of subsequent adverse events.
Acknowledgments
We would like to thank participating physicians and nurses for their contribution in conducting the registry. ACS Full Spectrum Registry Principle Investigators: Kuan-Chen Chang, China University Medical Hospital; Chia-Lin Chao, Taoyuan General Hospital, Department of Health; Yi-Jen Chen, Wan-Fang Hospital; Chien-Cheng Chen, Show Chwan Memorial Hospital; Cheng-Yun Chen, Chia-Yi Christian Hospital; Chung-Yin Chen, Kuang Tien General Hospital; Fu-Tien Chiang, National Taiwan University Hospital; Shao-Yueh Chiang, Cheng Ching Hospital; Li-Ping Chou, Sin Lau Hospital The Presbyterian Church of Taiwan; Ching-Chang Feng, Tainan Municipal Hospital; Charles Jia-Yin Hou, Mackay Memorial Hospital; Kwan-Li Hsu, E-Da Hospital; Tsuei-Yuan Huang, Chi-Mei Hospital; Gwo-Ping Jong, Taichung Armed Forces General Hospital; Yu-Lin Ko, Taipei Tzu Chi General Hospital; Wen-Ter Lai, Kaohsiung Medical University Chung-Ho Memorial Hospital; Wen-Lieng Lee, Taichung Veterans General Hospital; Chun-I Lee, Pingtung Christian Hospital; Meng-Huan Lei, Lo-Tung Po-Ai Hospital; Ai-Hsien Li, Far Eastern Memorial Hospital; Yi-Heng Li, National Cheng Kung University Hospital; Jou-Wei Lin, National Taiwan University Hospital, Yun-lin Branch; Tin-Kwang Lin, Dalin Tzuchi General Hospital; Jih-Min Lin, Kee-lung Hospital, Department of Health; Shing-Jong Lin, Taipei Veterans General Hospital; Hung-Shun Lo, Cathay General Hospital; Guang-Yuan Mar, Kaohsiung Veterans General Hospital; Chun-Ming Shih, Taipei Medical University Hospital; Kou-Gi Shyu, Shin Kong Wu Ho-Su Memorial Hospital; Cheng-Dao Tsai, Changhua Christian Hospital; Chuen-Den Tseng, National Taiwan University Hospital; Kwo-Chang Ueng, Chung Shan Medical University Hospital; Ji-Hung Wang, Hualien Tzu Chi General Hospital; Kuang-Te Wang, Mackay Memorial Hospital, Taitung Branch; Ming-Shien Wen, Linkou Chang Gung Memorial Hospital; Szu-Chi Wen, Hsin Chu General Hospital, Department of Health; Chiung-Jen Wu, Kaohsiung Chang Gung Memorial Hospital; Shih-Peng Yang, Tri-Service General Hospital; Wei-Hsian Yin, Cheng-Hsin Hospital.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported by the Sanofi-Aventis Taiwan Co. Ltd. and Bristol-Myers Squibb (Taiwan) Ltd. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rouleau JL, Talajic M, Sussex B, Potvin L, Warnica W, et al. (1996) Myocardial infarction patients in the 1990s–their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol 27: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong PW, Fu Y, Chang WC, Topol EJ, Granger CB, et al. (1998) Acute coronary syndromes in the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation 98: 1860–1868. [DOI] [PubMed] [Google Scholar]
- 3. Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, et al. (2013) Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J 77: 934–943. [DOI] [PubMed] [Google Scholar]
- 4. Boden H, van der Hoeven BL, Karalis I, Schalij MJ, Jukema JW (2012) Management of acute coronary syndrome: achievements and goals still to pursue. Novel developments in diagnosis and treatment. J Intern Med 271: 521–536. [DOI] [PubMed] [Google Scholar]
- 5. Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, et al. (2013) Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J 77: 439–446. [DOI] [PubMed] [Google Scholar]
- 6. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, et al. (2006) Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 333: 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, et al. (2000) The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 284: 835–842. [DOI] [PubMed] [Google Scholar]
- 8. Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, et al. (2000) Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 101: 2557–2567. [DOI] [PubMed] [Google Scholar]
- 9. Henriksson KM, Farahmand B, Johansson S, Asberg S, Terent A, et al. (2010) Survival after stroke–the impact of CHADS2 score and atrial fibrillation. Int J Cardiol 141: 18–23. [DOI] [PubMed] [Google Scholar]
- 10. Crandall MA, Horne BD, Day JD, Anderson JL, Muhlestein JB, et al. (2009) Atrial fibrillation significantly increases total mortality and stroke risk beyond that conveyed by the CHADS2 risk factors. Pacing Clin Electrophysiol 32: 981–986. [DOI] [PubMed] [Google Scholar]
- 11. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 12. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 13. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2011) 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 57: e101–198. [DOI] [PubMed] [Google Scholar]
- 14. Poci D, Hartford M, Karlsson T, Herlitz J, Edvardsson N, et al. (2012) Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest 141: 1431–1440. [DOI] [PubMed] [Google Scholar]
- 15. Shyu K-G, Wu C-J, Mar G-Y, Hou CJY, Li AH, et al. (2011) Clinical Characteristics, Management and In-Hospital Outcomes of Patients with Acute Coronary Syndrome - Observations from the Taiwan ACS Full Spectrum Registry. Acta Cardiol Sin 27: 10. [Google Scholar]
- 16. Park JH, Joung B, Son NH, Shim JM, Lee MH, et al. (2011) The electroanatomical remodelling of the left atrium is related to CHADS2/CHA2DS2VASc score and events of stroke in patients with atrial fibrillation. Europace 13: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 17. Chao TF, Cheng CC, Lin WS, Tsao HM, Lin YJ, et al. (2011) Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm 8: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 18. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2006) ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114: e257–354. [DOI] [PubMed] [Google Scholar]
- 19. John Camm A (2013) Managing anticoagulation for atrial fibrillation: current issues and future strategies. J Intern Med 273: 31–41. [DOI] [PubMed] [Google Scholar]
- 20. Gustafsson F, Kober L, Torp-Pedersen C, Hildebrandt P, Ottesen MM, et al. (1998) Long-term prognosis after acute myocardial infarction in patients with a history of arterial hypertension. TRACE study group. Eur Heart J 19: 588–594. [DOI] [PubMed] [Google Scholar]
- 21. Avezum A, Makdisse M, Spencer F, Gore JM, Fox KA, et al. (2005) Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J 149: 67–73. [DOI] [PubMed] [Google Scholar]
- 22. Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, et al. (2008) Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke 39: 2929–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herman B, Greiser E, Pohlabeln H (1997) A sex difference in short-term survival after initial acute myocardial infarction. The MONICA-Bremen Acute Myocardial Infarction Register, 1985–1990. Eur Heart J 18: 963–970. [DOI] [PubMed] [Google Scholar]
- 25. Hochman JS, McCabe CH, Stone PH, Becker RC, Cannon CP, et al. (1997) Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. TIMI Investigators. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol 30: 141–148. [DOI] [PubMed] [Google Scholar]
- 26. Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, et al. (1999) Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med 341: 226–232. [DOI] [PubMed] [Google Scholar]
- 27. Dormandy J, Heeck L, Vig S (1999) Lower-extremity arteriosclerosis as a reflection of a systemic process: implications for concomitant coronary and carotid disease. Semin Vasc Surg 12: 118–122. [PubMed] [Google Scholar]
- 28. Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, et al. (1996) Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 25: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 29. Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, et al. (1996) Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ 313: 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dormandy J, Heeck L, Vig S (1999) The natural history of claudication: risk to life and limb. Semin Vasc Surg 12: 123–137. [PubMed] [Google Scholar]
- 31. Al-Thani HA, El-Menyar A, Zubaid M, Rashed WA, Ridha M, et al. (2011) Peripheral arterial disease in patients presenting with acute coronary syndrome in six middle eastern countries. Int J Vasc Med 2011: 815902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldberg RJ, McCormick D, Gurwitz JH, Yarzebski J, Lessard D, et al. (1998) Age-related trends in short- and long-term survival after acute myocardial infarction: a 20-year population-based perspective (1975–1995). Am J Cardiol 82: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 33. Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, et al. (2002) Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med 136: 341–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.