Abstract
Rationale
Evidence from a growing body of literature suggests that alcohol, even at moderate dose levels, disrupts the ability to ignore distractors. However, little work has been done to elucidate the neural processes underlying this deficit.
Objective
The present study was conducted to determine if low-to-moderate alcohol doses affect sensory gating, an electrophysiological phenomenon believed to reflect the pre-attentive filtering of irrelevant sensory information.
Methods
Sixty social drinkers were administered one of three doses intended to produce breath alcohol concentrations of 0.0% (placebo), .04% (i.e., low dose), and .065% (i.e., moderate dose). A paired-click paradigm consisting of 100 pairs of identical tones (S1 and S2) was used to assess sensory gating. Amplitudes of the P50, N100 and P200 auditory evoked potentials (AEPs) were used to calculate gating difference (S1 – S2) and ratio (S2/S1) scores.
Results
The moderate alcohol dose significantly decreased P50 and N100 gating relative to placebo. Comparisons between the difference and ratio scores helped characterize the gating mechanisms affected at these stages of information processing. Alcohol did not alter P200 sensory gating.
Conclusions
These data suggest that alcohol disrupts pre-attentional sensory filtering processes at BrACs below the current .08% legal limit. Future studies should perform a combined assessment of sensory gating and selective attention to better understand the relationship between these two alcohol-induced deficits.
Keywords: Auditory evoked potentials, Sensory gating, Moderate alcohol, Attention
Introduction
Despite the reported health benefits of a moderate drinking lifestyle (e.g., Fuller, 2011), significant morbidity and mortality has been associated with the acute consumption of low-to-moderate alcohol dose levels (Kuendig et al., 2008; Taylor & Rehm, 2012). Laboratory studies conducted to explore possible neurocognitive deficits responsible for these consequences have identified an impaired ability to ignore irrelevant and potentially distracting stimuli. For example, Fillmore and colleagues (2000) reported a disruption of the negative priming effect at a breath alcohol concentration (BrAC) of 0.065%, indicating an inability to suppress the processing of previously presented task-irrelevant distractors. Similar impairments in the ability to ignore irrelevancy have also been observed using inhibition of return and delayed ocular response tasks at or below the same BrAC level (Abroms & Fillmore, 2004; Abroms et al., 2006).
Although the acute effects of alcohol on attention have been extensively examined, its effects on pre-attentive mechanisms operating at earlier stages of information processing have remained relatively unexplored. Investigation of these pre-attentive mechanisms would be of value considering the influence exerted by stimulus driven, bottom-up processes on top-down cognitive and behavioral functions such as selective attention (Corbetta & Shulman, 2002). Based on the clinical observations of schizophrenic patients, Venables (1964) proposed that an inability to ignore irrelevancy in the environment might result from a dysfunctional pre-attentive filter that restricts the access of irrelevant stimuli to higher-order cognitive centers. By preventing sensory overload, or flooding, this sensory filter allows unimpaired individuals to cope with large amounts of information without taxing attentional resources.
In more recent investigations, this filtering mechanism has been operationalized as a neurophysiological phenomenon referred to as sensory gating. One common method to test sensory gating involves the use of a paired-click paradigm which takes advantage of stimulus novelty to measure the brain’s response to irrelevant stimuli. Using the orienting response as a behavioral marker of stimulus salience, novel stimuli have been shown to be highly salient, reliably evoking a reorientation of attention. However, redundancy in the presentation of a stimulus decreases orientation towards it, indicating a reduction in its salience (Neo & Chua, 2006; Siddle et al., 1983). During the paired-click paradigm, pairs of identical tone stimuli are presented to an individual while simultaneously recording electroencephalographic (EEG) activity (Adler et al., 1982). The presentation of each tone elicits a set of three auditory-evoked potentials (AEPs) reflecting sequential stages of information processing: the P50, N100, and P200. Sensory gating is observed when the redundant second tone in each pair elicits an attenuated response (i.e., smaller amplitudes of the AEPs) compared to the contextually relevant, novel first tone.
To date, the majority of the evidence linking sensory gating and attentional functions has come from clinical populations. For example, both schizophrenics (Adler et al., 1982; Freedman et al., 1987) and patients suffering from dorsolateral prefrontal cortex lesions (Knight et al., 1999), two populations characterized by high levels of distractibility and noted deficits in attention, exhibit impaired sensory gating. Importantly, an individual’s level of sensory gating has been found to correlate with their performance on certain attention tasks among both patients (Hsieh et al., 2004; Smucny et al, 2013) and healthy controls (Lijffijt et al., 2009a; Yandon et al., 2009).
The increased use of novel techniques to measure gating may also further elucidate potential relationships with attentional processes. Because difference and ratio scores derived from AEP amplitudes have been found to reflect different aspects of the gating process (e.g. sensory registration versus suppression [Brokhaus-Dumke et al, 2008]), both are now commonly reported as measurements of sensory gating. The use of the N100 and P200 components to evaluate gating in addition to the more traditional P50 component has also become more frequent due to their superior reliability (Anokhin et al., 2007; Rentzsch et al., 2008).
Beyond impairments in the ability to ignore task-irrelevant distractors, there are additional reasons to believe that alcohol consumption might be associated with impaired sensory gating processes. For example, functional neuroimaging studies have revealed reductions in blood flow to and activity of prefrontal cortical regions believed to be critical for robust sensory gating (Trim et al., 2010; Wendt & Risberg, 2001). In vitro, ethanol has been shown to inhibit the activity of nicotinic receptor subtypes (Yu et al., 1996) previously implicated in proper P50 sensory gating. Furthermore, preliminary evidence suggests that the chronic alcohol abuse observed in alcoholics results in impaired P50 sensory gating (Marco et al., 2005). In an acute setting, alcohol consumption was also associated with reduced levels of P50 gating in a previous investigation of 12 social drinkers (Freedman et al., 1986). However, a poorly controlled alcohol administration protocol (participants were instructed to drink white wine to their usual level of intoxication), a small sample size, and the lack of N100 and P200 data underscore the need for a more systematic evaluation.
To address the unresolved issues of this previous investigation and extend our understanding of low-to-moderate dose effects on sensory processing, the present study examined the effects of controlled alcohol administration on P50, N100, and P200 sensory gating in a group of 60 community dwelling moderate drinkers. Both ratio and difference score measures of amplitude suppression were calculated from data collected during a paired-click paradigm. Based on the results of the Freedman et al. study as well as the previously reported deficits in attention, we predicted impaired gating of all three AEPs following the acute administration of low-to-moderate alcohol doses.
Methods
Exclusion Criteria
60 (34 females) participants between the ages of 25 and 55 (31.4±8.9) were included in the study. Participants were excluded if they 1) were not moderate drinkers (typical consumption of ≤1 drink/day for women, ≤2 drinks/day for men (USDA/USDHHS, 2010) and at least 1 drink/month), 2) were smokers, 3) had a first degree relative diagnosed with schizophrenia, 4) had a hearing threshold above 10 dB, 5) were color blind, 6) met criteria for a current or past Axis I psychiatric disorder, or 7) suffered from a medical condition or were taking a medication that either contraindicated the use of alcohol or alters electrophysiological or neurocognitive functioning (e.g. benzodiazepines).
Screening Procedures
Participants were recruited via flyers, newspaper, and radio advertisements. Interested participants underwent an initial screening session. Demographic information, general intellectual abilities (Shipley Institute of Living Verbal (SIL-V) and Abstracting (SIL-A) [Zachary, 1986]), substance use histories, current alcohol use patterns (Quantity-Frequency Index (QFI) [Cahalan et al., 1969] and Max-QFI), brief medical histories (including current medications), affective state (Beck Depression Inventory (BDI-II) [Beck et al., 1996] and Spielberger Anxiety Inventory (STAI) [Spielberger, 1983]), and family histories of substance abuse and psychiatric illness were collected. QFI and Max-QFI provide an estimate of the average and maximum amount of absolute ethanol (oz.) consumed per day over the past six months, respectively. A computerized clinical research interview based on DSM-IV (American Psychiatric Association, 1994) criteria (computerized Diagnostic Interview Schedule (cDIS); [Robins et. al., 1995]) was also used to establish Axis I disorder diagnoses. At the end of the screening session, height/weight, auditory thresholds (MA-25 [MAICO Diagnostics, Minneapolis, MN]), and visual acuity (i.e., Snellen chart) were examined.
All participants provided written consent prior to the collection of any data and were compensated for their time. All procedures were approved by the University of Florida Medical Institutional Review Board.
Laboratory Protocol
Participants were instructed to abstain from alcohol for 24 hrs and fast for a minimum of 4 hrs prior to testing and transported to our lab at 9:30 AM. After providing consent, they were given a small breakfast snack (~220 Cal). Following breakfast, research assistants collected a breath and urine samples to ensure sobriety and exclude concurrent use of substances with the potential to alter electrophysiological indices. Pregnancy screens were also performed for all females prior to beverage administration. No individuals were excluded based on a positive alcohol, toxicology, or pregnancy test.
Alcohol Administration
Participants were randomly assigned within sex to one of the three dose groups. Those participants assigned to one of the two active dose conditions received a volume of 100% medical grade ethanol intended to produce a peak breath alcohol concentration (BrAC) of either .04% (low dose) or .065% (moderate dose) according to the revised Widmark Equation (Watson, 1980). This dose of ethanol was split into two beverages, each combined with 183 ml of sugar and caffeine free lemon-lime soda. The placebo dose consisted of two beverages containing 183 ml of the same soda. These beverages were misted with a negligible amount of ethanol to enhance placebo effectiveness. All participants were given 5 min to consume both beverages. Subjective ratings of intoxication (10-point Likert scale) and BrACs (Intoxilyzer, Model 400; CMI, Inc., Owensboro, KY) were recorded at 15, 30, 40, 55, and 65 min following the consumption of this beverage.
A booster beverage was administered 40 min following the consumption of the original dose to ensure that participants achieved their intended peak BrAC level. If the participant's BrAC at this time-point was greater than 50% of their target (i.e., .02% for the low dose and .0325% for the moderate dose), the booster consisted of 183 ml of soda misted with a negligible amount of ethanol. If their BrAC was below 50% of their target, the booster consisted of half of their original ethanol dose in addition to the requisite amount of soda needed to bring the total beverage volume to 183 ml. Participants in the placebo condition always received a placebo booster.
Recording Procedures
All electrophysiological recordings were conducted in a sound-attenuated, electrically shielded booth (Eckel Industries of Canada Limited, Morrisburg, Ontario). Participants were fitted with an elastic cap containing a 64 electrode array in an expanded international 10/20 system (Electro-Cap International, Inc., Eaton, OH). Linked earlobe electrodes were used as a reference with a mid-forehead ground. Two electrodes were also placed above and below the outer canthus of the left eye. Impedances were maintained below 10 kΩ. NeuroScan 4.4 Acquire (Compumedics USA, Charlotte, NC) was used to record continuous electroencephalography (EEG). The amplifier was set to a gain of 10,000x. Data were subjected to an analog .15 – 50 Hz band-pass filter (6 dB/oct). Data were sampled at a rate of 500 Hz.
Paired-Click Paradigm
Participants were seated upright 70 cm from a computer monitor with their chins in a chinrest. 100 pairs (S1 and S2) of identical tone stimuli (4 ms clicks presented at 85 dB over a 55 dB white-noise background) were presented to participants over two trial blocks separated by a 2 min break period. Stimuli were presented through calibrated earplugs (ER-3 Tuberphone, Etimotic Research, Elk Grove Village, IL) using STIM software (Neuroscan, Inc., El Paso, TX). An interstimulus interval (ISI) of 500 ms occurred between the tones in each pair and an average intertrial interval of 8 sec (6 – 10 sec range) occurred between pairs. A fixation "+" was presented in the center of the monitor throughout the testing session. Participants were instructed to keep their gaze fixated on the "+" while they passively listened to the tones as they were presented.
Two task orders, counter-balanced within dose group, were used during the protocol. The paired-click paradigm was administered at either 15 or 40 min post-consumption. BrACs and subjective ratings of intoxication were recorded during the 2 min break between trial blocks of the paired-click paradigm.
Peak Identification
EEG data were processed off-line using EEGLAB Toolbox (Delorme & Makeig, 2004). Separate filtering parameters were used for optimal visualization of the P50 (10 – 55 Hz; 24 dB/oct) and N100 and P200 (.3 – 30 Hz; 24 dB/oct) components. Epochs ranged from 100 ms prior to each tone stimulus (baseline) to 398 ms following it. Epochs containing voltage recordings larger than ±150 uV were rejected. This approach resulted in an average of 99.7±1.3 and 91.4±12.5 usable tone pairs for the P50 and N100-P200 components respectively. The number of epochs rejected did not differ between the three dose groups (p’s>.1). The data were then subjected to independent component analysis (ICA) (Jung et al., 2001) followed by an automated technique for identifying and removing artifacts (ADJUST) (Mognon et al., 2011). S1 and S2 epochs were averaged separately and baseline corrected.
P50, N100, and P200 peaks were automatically detected (EEGLAB Toolbox) using data from the Cz electrode (P50: most positive local peak (±4 data points) 45 – 90 ms post-stimulus; N100: most negative local peak 70 – 170 ms post-stimulus; P200: most positive local peak 125 – 250 ms post-stimulus). Consistent with previous work (e.g., Tricht et al., 2012) the N40 (i.e., most negative local peak preceding the P50) was used to calculate the P50 peak-to-peak amplitude measure. The N100 and P200 amplitudes were measured relative to pre-stimulus baselines. A participant’s data were excluded from analysis if a S1 evoked amplitude was not identifiable within the time windows or if technical problems were encountered during the recording procedure. This approach resulted in the exclusion of four participants from our analysis of all three AEPs as well as a fifth who was excluded only from the P50 analysis. As an additional constraint, S2 evoked potentials had to occur ±15, ±40, and ±80 ms of the S1 evoked P50, N100, and P200 components respectively (Lijffijt et al., 2009a). S2 components were assigned amplitudes of 0 uV and assumed to be completely attenuated if they did not occur within these time windows (Negamoto et al, 1989). Both ratio (S2/S1) and difference scores (S1 – S2) were calculated for each component. Larger difference scores and smaller ratio scores are indicative of more robust sensory gating. When S2 amplitude was > S1, ratio and difference scores were capped at 1 and 0 uV respectively to represent a complete absence of sensory gating (Cadenhead et al., 2000).
Statistical Analysis
Demographic and alcohol use data collected during the screening session were subjected to one-way ANOVAs to explore potential differences between dose groups.
Separate 3 (dose) X 2 (sex) X 2 (order) ANOVAs were constructed to explore effects of sex, task order, and their potential interaction with alcohol dose on P50, N100, and P200 sensory gating. Sex and task order were dropped from the model because neither main effects nor interactions with alcohol dose were found for either factor on any of the gating measures (p’s>.05).
Skewness and kurtosis values were used to examine deviations from normality for each gating measure (Tabachnick & Fidell, 1989). One-way ANOVAs were used to test the effects of alcohol dose on sensory gating for all normally distributed gating measures. A Kruskal-Wallis analysis was used to examine dose effects if the distribution of a gating measure exhibited significant skewness or kurtosis. Significant and trend-level (i.e. p≤0.10) dose effects were characterized by comparing sensory gating scores between groups using t-tests and Wilcoxon-Mann-Whitney tests for normally and non-normally distributed variables, respectively. Corrections for multiple comparisons (Tukey’s for normally distributed variables and Bonferroni’s for variables exhibiting skewness or kurtosis) were applied to all post-hoc tests to control for Type I error.
S1 and S2 evoked amplitude measures were included in separate one-way ANOVAs to determine if the effects of alcohol on gating were primarily driven by dose group differences in the response to either the S1, S2, or both. Correlations between these amplitude measures and the sensory gating scores for each evoked potential were also performed. This analysis was conducted to explore the relative contributions of S1 and S2 alcohol related effects to the overall sensory gating measures.
Pearson correlations were performed between BrACs collected during the 2 min break between trial blocks and measures of sensory gating for those participants who received an active alcohol dose. This approach was taken to allow for comparisons with previous literature (e.g., Freedman et al., 1986) and to provide further validation for our dose group results. In addition, correlations between P50, N100, and P200 gating measures were conducted to explore the relationship between gating processes at the three AEPs. SAS Version 9.3 (SAS Institute, Inc., Cary, NC) was used for all statistical operations.
Results
Demographics
Demographic and alcohol use data for individuals in the three dose groups are presented in Table 1. No differences between dose groups were observed for any variable of interest (p’s>.1).
Table 1.
Demographic data by alcohol dose condition.
| Placebo (n=20) Mean(SD) |
0.04% (n=20) Mean (SD) |
0.065% (n=20) Mean (SD) |
|
|---|---|---|---|
| Age | 32.10 (8.7) | 30.30 (8.1) | 31.90 (10) |
| Female, % | 55 | 55 | 60 |
| Education | 16.30 (1.8) | 16.10 (3.9) | 17.50 (1.9) |
| BDI-IIa | 01.47 (2.3) | 02.75 (2.8) | 03.30 (3.2) |
| STAIb | 41.20 (5.1) | 39.85 (5.4) | 41.75 (6.5) |
| QFIc | 00.28 (0.3) | 00.37 (0.3) | 00.35 (0.3) |
| Max-QFI | 03.17 (1.3) | 03.37 (1.8) | 03.28 (2.0) |
| BMId | 25.58 (4.7) | 25.68 (4.8) | 25.02 (3.9) |
Beck Depression Inventory-II (Beck et al, 1996);
Spielberger State Anxiety Inventory (Spielberger, 1983);
Quantity-frequency Index (Cahalan et al, 1969);
Body-Mass Index
Breath Alcohol Concentration and Subjective Intoxication
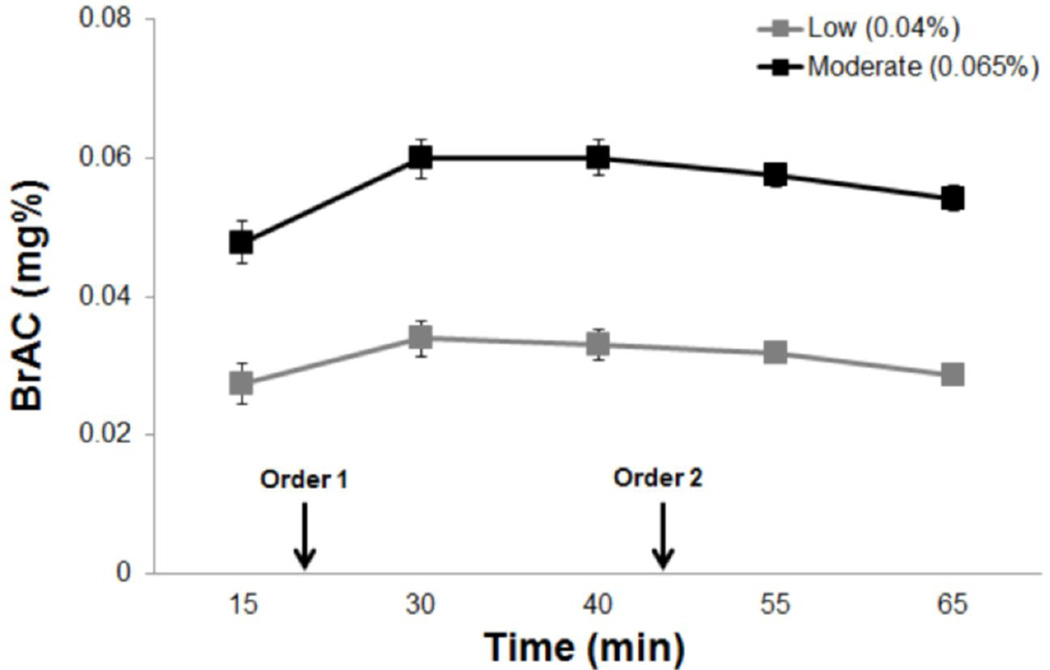
As expected, participants in the moderate dose group recorded significantly higher BrACs than those in the low dose group (F1,37=108.88, p<.01) (Figure 1). Participants in the low and moderate dose groups recorded BrACs of .036%±.009 and .058%±.011, respectively, during the paired-click paradigm (Figure 1). There was no interaction between time-point and dose group on BrAC (p>.1).
Figure 1.
Breath alcohol concentration (BrAC) curves for the individuals receiving the low and moderate alcohol doses. Arrows indicate the beginning of the paired-click paradigm for participants assigned to either task order 1 and 2. Error bars represent SEM.
One female in the low dose group was the only participant to receive an active booster beverage. Her BrAC at the time of the paired-click paradigm and sensory gating values did not differ significantly from those of her dose group (p’s>.2).
P50 Component
Difference Score (S1 – S2)
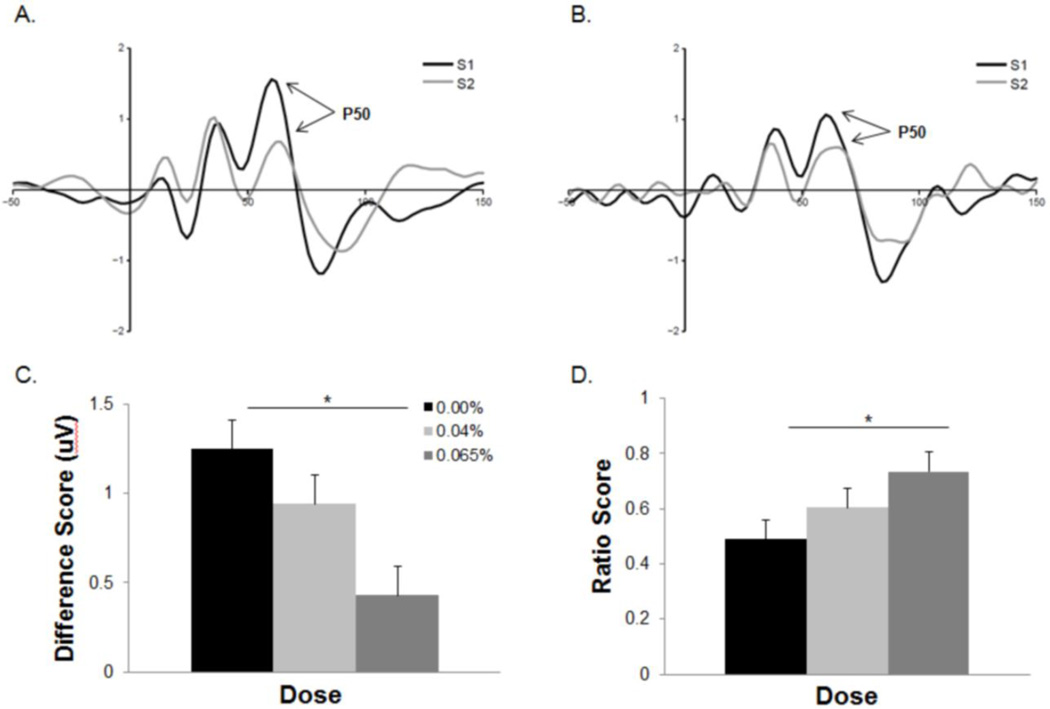
Alcohol dose had a significant effect on the P50 difference gating score (F2,52=6.52, p<.01). Participants receiving the moderate dose recorded significantly smaller P50 difference scores relative to placebo (t36=3.58, p<.01, d=1.19) (Figure 2). No other dose group comparisons yielded a significant difference (p’s>.05). P50 difference scores were significantly correlated with BrAC measures recorded during the inter-block break period (r=−.35, p=.04).
Figure 2.
Averaged P50 waveforms for participants in the placebo (A) and moderate (B) dose groups. The moderate dose group exhibited significantly smaller difference scores (C) and larger ratio scores (D) than the placebo group. *p<.05. Error bars represent SEM.
There was a significant effect of dose on S1-P50 amplitude (F2,52=3.19, p=.05). Participants in the moderate dose group exhibiting significantly smaller amplitudes than those in in the placebo condition (t36=2.47, p=.04, d=0.82) (Table 2). S1-P50 amplitudes were significantly correlated with P50 difference scores (r=.63, p<.01). No dose effect was observed for S2-P50 amplitude (p>.9) and S2 amplitude was not associated with P50 difference scores (p>.5).
Table 2.
Amplitude (uV) and latency (ms) 1 of the P50, N100, and P200 components in response to S1 and S2
| Placebo | 0.04% | 0.065% | ||||
|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | |
| P50 Amp | 2.58 (0.3)* | 1.50 (0.2) | 2.29 (0.3) | 1.48 (0.2) | 1.62 (0.2)* | 1.44 (0.2) |
| P50 Lat | 63.29 (2.7) | 63.87 (2.4) | 60.26 (1.6) | 62.31 (2.5) | 61.27 (1.7) | 65.97 (1.9) |
| N100 Amp | 8.39 (0.8) | 4.39 (0.5) | 6.61 (0.7) | 4.72 (0.5) | 6.63 (0.8) | 4.50 (0.6) |
| N100 Lat | 99.66 (4.4) | 88.65 (2.8) | 94.22 (2.5) | 98.20 (4.0) | 94.56 (3.3) | 94.25 (3.1) |
| P200 Amp | 18.01 (1.5)* | 6.19 (0.6) | 14.93 (1.2) | 5.98 (0.6) | 12.03 (1.5)* | 5.27 (0.9) |
| P200 La | 203.0 (6.0) | 184.8 (7.3) | 193.9 (7.2) | 182.2 (6.8) | 202.8 (6.5) | 191.84 (9.3) |
Significant differences between dose groups (p<.05)
Ratio Score (S2/S1)
Alcohol dose had a significant effect on P50 ratio scores (F2,52=3.04, p=.05). Participants in the moderate dose group recorded larger ratio scores than those in the placebo group (t36=2.47, p=.04, d=.0.82). No other dose group effects on P50 ratio score were observed (p’s>.4). P50 ratio scores were not correlated with BrACs (p>.3). S2-P50 amplitudes (r=.50, p<.01), but not S1-P50 amplitudes (p>.3), were correlated with P50 ratio gating scores.
N100 Component
Difference Score (S1 – S2)
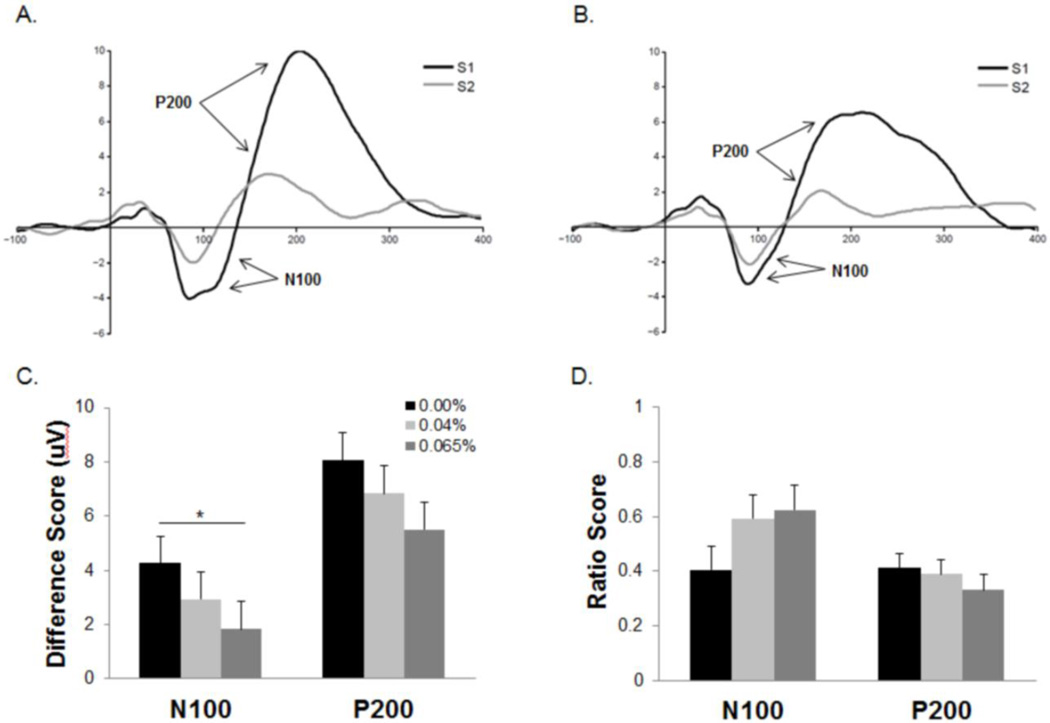
A Kruskal-Wallis analysis was applied to the N100 difference score because the distribution of this measure exhibited significant skewness and kurtosis. There was a trend-level effect of dose group on N100 difference gating scores (X2=5.22, p=.07). Participants receiving the moderate dose exhibited significantly larger difference scores than those in the placebo condition (z=2.42, p=.016) (Figure 3). No other group differences yielded a significant difference (p’s>.2). N100 difference scores were significantly correlated with BrAC measures recorded during the inter-block break period (r=−.36, p=.03).
Figure 3.
Averaged N100-P200 waveforms for participants in the placebo (A) and moderated (B) dose groups. Smaller N100 gating difference scores (C) were observed among individuals in the moderate dose group compared to those receiving a placebo beverage. No effect of dose was observed on the N100 ratio score (D) or either P200 gating measure. *p<.05. Error bars represent SEM.
Dose group did not have an effect on the N100 amplitude elicited by either S1 or S2 (p’s>.05) (Table 2). Both S1-N100 (r=.73, p<.01) and S2-N100 (r=−.35, p<.01) amplitudes were correlated with N100 difference scores.
Ratio Score (S2/S1)
There was no effect of alcohol dose on N100 ratio gating score (p>.1) nor did this measure correlate with BrACs recorded during testing (p>.2). However, both S1-N100 (r=.45, p<.01) and S2-N100 (r=−.55, p<.01) amplitudes were correlated with the N100 ratio gating score.
P200 Component
Difference Score (S1 – S2)
Alcohol dose did not affect P200 difference scores (p>.2) (Figure 3). No correlations were observed between P200 difference scores and this BrAC measure (p’s>.8).
Dose group did, however, have a significant effect on S1-P200 amplitude (F2,54=3.53, p=.04). Participants receiving the moderate dose recorded significantly smaller S1-P200 components relative to placebo (t36=2.65, p=.03, d=0.88). No other group differences were observed for S1-P200 amplitudes (p>.2). S2-P200 amplitude did not differ between dose groups (p’s>.2) (Table 2). There was a significant correlation between S1-P200 amplitudes and P200 difference scores (r=.87, p<.01). However, S2-P200 amplitudes were not associated with P200 difference scores (p>.6).
Ratio Score (S2/S1)
Alcohol dose had no effect on P200 ratio gating scores (p>.5). There was no correlation between BrACs and P200 ratio scores (p>.4). Both S1-P200 (r=−.36, p<.01) and S2-P200 (r=.49, p<.01) amplitude measures were correlated with P200 ratio scores.
Gating Score Correlations
P50 and N100 difference scores were significantly correlated (r=.52, p<.01). Neither measure, however, was correlated with P200 difference scores (p’s>.05).
P50 and N100 ratio scores were also significantly correlated (r=.31, p=.03). Again, neither one was correlated with P200 ratio scores (p>.1).
Discussion
Sensory gating is an electrophysiological phenomenon believed to represent a mechanism for filtering irrelevant sensory information and promoting attentional efficiency. The present study investigated the effects of low-to-moderate alcohol doses on sensory gating of the P50, N100, and P200 auditory evoked potentials in social drinkers. The moderate dose produced deficits in P50 and N100 gating. P200 sensory gating, however, was not significantly affected by either dose. In addition, the moderate dose resulted in reduced P50 and P200 S1 amplitudes relative to the placebo beverage. These findings suggest that alcohol disrupts pre-attentional sensory filtering and registration processes at BrACs below the current .08% legal limit and extend the findings of a previous study which observed an association between BrAC and P50 sensory gating (Freedman et al., 1986).
The effect of alcohol on N100 sensory gating, however, was only observed using the difference score. This contrast between N100 difference and ratio scores may be related to differences in their reliability (Dalecki et al., 2011; Rentzsch et al., 2008) and/or sensitivity to alcohol effects. It is also possible, however, that these two measures reflect different aspects of sensory gating. Previous research suggests the difference score is more sensitive to alterations in S1 evoked amplitudes and are therefore more closely associated with sensory registration or “gating-in” functions whereas ratio scores are more reflective of S2 suppression or “gating-out” mechanisms (Brokhaus-Dumke et al, 2008). This theory is supported by the pattern of correlations between amplitude measures and gating indices observed in the current study across all three components (i.e., stronger correlations between S1, compared to S2, amplitudes and difference scores, with the opposite being true for ratio scores). Therefore, our N100 data provide evidence for a predominant “gating-in” deficit induced by moderate alcohol doses.
This deficit in sensory registration is in line with previous reports of an impaired mismatch negativity (MMN; an electrophysiological response to relevant, deviant auditory stimuli) following the consumption of moderate alcohol doses (He et al, 2013; Kenemans et al, 2010). While reduced P50 difference scores as well as S1-P50 and S1-P200 amplitudes observed in the moderate dose group also support this possibility, the P50 ratio scores suggest alcohol also disrupts inhibitory, “gating-out” processes at earlier stages of information processing. Importantly, a post-hoc analysis of our sensory gating data revealed that the observed gating deficits occur independent of disruptions in sensory registration. The moderate dose group continued to exhibit significantly smaller difference scores and larger ratio scores compared to placebo when S1-P50 amplitude was included as a covariate (p’s<.05).
Although the neurobiological processes underlying these gating deficits remain unclear, ethanol’s inhibition of alpha-7 nicotinic acetylcholine receptor function (Yu et al., 1996) might represent a potential mechanism. Work in both animals (Feuerbach et al., 2009; Stevens et al., 1996) and humans (Freedman et al., 1997; Raux et al., 2002) suggest this receptor is critical for proper P50 sensory gating. Alcohol’s disruption of dorsolateral prefrontal cortical functioning during neurocognitive task performance (Paulus et al., 2006; Wendt & Risberg, 2001) may provide an additional explanation for the observed impairments. Recent studies using functional neuroimaging modalities lend support to the emerging theory that the lateral prefrontal cortex plays a critical role in P50 sensory gating (Ehlis et al., 2009; Mayer et al., 2009). These findings are in line with observations from studies examining disrupted sensory gating in populations suffering from impaired prefrontal functions (i.e., schizophrenic and lesion patients).
Finally, these data provide further evidence that gating of the P50, N100, and P200 components reflect distinct phenomena. Despite a common susceptibility to alcohol effects and significant correlations between P50 and N100 gating indices, gating scores for neither component correlated with P200 gating which was not affected by alcohol dose. Furthermore, alcohol appears to disrupt P50 and N100 gating via different mechanisms as evidenced by the differential effect of the moderate dose on S1 evoked amplitudes and ratio scores. A possible explanation for the discord between the sensory gating data of the three AEPs could be their different susceptibilities to endogenous attentional states. For example, N100 amplitude increases with attention to the stimulus (Hillyard et al., 1973; Lee et al, 2013) whereas the opposite is true for the P200 (Crowley & Colrain, 2004). In addition, attentional manipulations have been shown to affect N100 and P200, but not P50, sensory gating values (Jerger et al., 1992; Kho et al., 2003; Rosburg et al., 2009). Therefore, the three gating measures may exhibit little to no relationship depending on the attentional resources allocated to the tone stimuli by the participant. Although an effort was made to control for this variable (i.e., instructions to passively listen to tones), the attentional state of the participants could not be confirmed.
Limitations
The potential effect of BrAC limb on sensory gating was not adequately addressed in the present investigation. There is an extensive literature documenting the phenomenon of acute tolerance (e.g., greater impairment observed during the ascending limb of the BrAC curve than the descending limb despite equivalent BrACs) (Schweizer & Vogel-Sprott, 2008). The present study, however, was not adequately designed to address possible effects of acute tolerance as sensory gating was tested at only one time-point in each participant. Instead, task order, which did not have an effect on gating measures, provided the best estimate of limb effects as the BrACs recorded during the task did not differ between order assignments (p>.8).
The variability in both behavioral and functional brain responses to alcohol among social drinkers also represents a potential limitation due to the between-subjects design of the current study (Paulus et al., 2012; Tolentino et al., 2011). However, the strict inclusion criteria applied and the absence of group differences in consumption variables (i.e. QFI and Max-QFI) mitigate the potential impact of baseline differences between dose groups. Furthermore, the between-subjects design avoids issues related to attrition and practice effects.
Conclusion
The data presented in this paper provide evidence that alcohol produces deficits in sensory mechanisms which promote the efficient allocation of attentional resources at BrACs as low as .06%. Specifically, the observed impairments in stimulus suppression and sensory registration suggest increases in distractibility and inattention at this dose level, respectively. Unlike impairments in motor and cognitive functions frequently observed at higher doses, individuals are unlikely to be aware these subtle pre-attentive deficits and, therefore, are less likely to be able to compensate for them. The effect of alcohol on sensory gating, however, was not observed uniformly across the electrophysiological measures tested. These findings suggest that the neural processes underlying P50, N100, and P200 gating and the sensory processing functions they subserve are distinct. Further work is needed to explore the relationships between the gating of these three components and the specific aspects of information processing they represent. In addition, a combined analysis of these electrophysiological findings with behavioral data would allow us to determine if deficits in sensory gating are mediating the alcohol-induced attentional impairments. This approach would be of value to areas of research beyond alcohol by identifying potential neural mechanisms underlying deficits of inhibitory attention that can be observed across clinical populations. Finally, future studies exploring interactions between smoking and alcohol consumption would be of value due to the frequent co-occurrence of both activities and the established role of nicotinic receptors in sensory gating.
Acknowledgments
Support for this project was provided by R01AA019802 (S.J. Nixon, PI); F30AA021315 (A.L. Sklar, Trainee; S.J. Nixon, Sponsor).
A special thanks to the members of the Nixon Lab team (Jeff Boissoneault, PhD, Ben Lewis, PhD, Robert Prather, Layla Lincolon, Lauren Hoffman, and Cole McCarty) who assisted in the collection of the data.
Footnotes
Neither of the authors maintains any financial agreements that would constitute a conflict of interest.
References
- Abroms BD, Fillmore MT. Alcohol-induced impairment of inhibitory mechanisms involved in visual search. Experimental and Clinical Psychopharmacology. 2004;12(4):243–250. doi: 10.1037/1064-1297.12.4.243. [DOI] [PubMed] [Google Scholar]
- Abroms BD, Gottlob LR, et al. Alcohol effects on inhibitory control of attention: distinguishing between intentional and automatic mechanisms. Psychopharmacology. 2006;188(3):324–334. doi: 10.1007/s00213-006-0524-y. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, et al. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological psychiatry. 1982;17(6):639–654. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV. Washington, DC: The Association; 1994. [Google Scholar]
- Anokhin AP, Vedeniapin AB, et al. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophr Res. 2007;89(1–3):312–319. doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, et al. Beck Depression Inventory, Second Edition. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first episode, and chronic patients. Biological psychiatry. 2008;64(5):376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, et al. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. The American journal of psychiatry. 2000;157(1):55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cissin L, et al. American Drinking Practices: A National Study of Drinking Behaviors and Attitudes (Monograph No. 6) New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews. Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clinical Neurophysiology. 2004;115(4):732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Dalecki A, Croft RJ, et al. An evaluation of P50 paired-click methodologies. Psychophysiology. 2011;48(12):1692–1700. doi: 10.1111/j.1469-8986.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ehlis AC, Ringel TM, et al. Cortical correlates of auditory sensory gating: a simultaneous near-infrared spectroscopy event-related potential study. Neuroscience. 2009;159(3):1032–1043. doi: 10.1016/j.neuroscience.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, et al. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56(1):254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Dixon MJ, et al. Alcohol affects processing of ignored stimuli in a negative priming paradigm. Journal of studies on alcohol. 2000;61(4):571–578. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophrenia bulletin. 1987;13(4):669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Waldo MC, et al. Electrophysiological effects of low dose alcohol on human subjects at high altitude. Alcohol and drug research. 1986;6(4):289–297. [PubMed] [Google Scholar]
- Fuller TD. Moderate alcohol consumption and the risk of mortality. Demography. 2011;48(3):1105–1125. doi: 10.1007/s13524-011-0035-2. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Ceballos NA, et al. Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. Journal of studies on alcohol and drugs. 2009;70(2):242–252. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Li B, et al. Effects of alcohol on auditory pre-attentive processing of four sound features: evidence from mismatch negativity. Psychopharmacology. 2013;225(2):353–360. doi: 10.1007/s00213-012-2816-8. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, et al. Electrical signs of selective attention in the human brain. Science. 1973;182(4108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Liu K, et al. Memory impairment and auditory evoked potential gating deficit in schizophrenia. Psychiatry research. 2004;130(2):161–169. doi: 10.1016/j.pscychresns.2002.12.001. [DOI] [PubMed] [Google Scholar]
- Jerger K, Biggins C, et al. P50 suppression is not affected by attentional manipulations. Biological psychiatry. 1992;31(4):365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, et al. Imaging Brain Dynamics Using Independent Component Analysis. Proceedings of the IEEE. 2001;89(7):1107–1122. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenemans JL, Hebly W, et al. Moderate alcohol disrupts a mechanism fordetection of rare events in human visual cortex. Journal of psychopharmacology. 2010;24(6):839–845. doi: 10.1177/0269881108098868. [DOI] [PubMed] [Google Scholar]
- Kho KH, Verkes RJ, Eling P, Zwarts MJ, Ellenbroek B, van Luijtelaar G. P50 gatiing is not affected by selective attention. Journal of Psychophysiology. 2003;17:23–29. [Google Scholar]
- Knight RT, Staines WR, et al. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta psychologica. 1999;101(2–3):159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kuendig H, Hasselberg M, et al. Acute alcohol consumption and injury: risk associations and attributable fractions for different injury mechanisms. Journal of studies on alcohol and drugs. 2008;69(2):218–226. doi: 10.15288/jsad.2008.69.218. [DOI] [PubMed] [Google Scholar]
- Lee AK, Larson E, et al. Using neuroimaging to understand the cortical mechanisms of auditory selective attention. Hearing research. doi: 10.1016/j.heares.2013.06.010. (Epub ahead of print). http://dx.doi.org/10.1016/j.heares.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, et al. Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alcoholism, clinical and experimental research. 2013;37(6):941–951. doi: 10.1111/acer.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Moeller FG, Boutros NN, Burroughs S, Lane SD, Steinberg JL, Swann AC. The role of age, gender, education, and intelligence in P50, N100, and P200 auditory sensory gating. Journal of Psychophysiology. 2009a;23(2):52–62. doi: 10.1027/0269-8803.23.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Lane SD, et al. P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46(5):1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco J, Fuentemilla L, et al. Auditory sensory gating deficit in abstinent chronic alcoholics. Neurosci Lett. 2005;375(3):174–177. doi: 10.1016/j.neulet.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Hanlon FM, et al. The neural networks underlying auditory sensory gating. Neuroimage. 2009b;44(1):182–189. doi: 10.1016/j.neuroimage.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48:229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, et al. Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biological psychiatry. 1989;25(5):549–561. doi: 10.1016/0006-3223(89)90215-1. [DOI] [PubMed] [Google Scholar]
- Neo G, Chua FK. Capturing focused attention. Perception & Psychophysics. 2006;68(8):1286–1296. doi: 10.3758/bf03193728. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, et al. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcoholism, clinical and experimental research. 2006;30(8):1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Schuckit MA, et al. High versus low level of response to alcohol: evidence of differential reactivity to emotional stimuli. Biological Psychiatry. 2012;72(10):848–855. doi: 10.1016/j.biopsych.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux G, Bonnet-Brilhault F, et al. The-2 bp deletion in exon 6 of the 'alpha 7-like' nicotinic receptor subunit gene is a risk factor for the P50 sensory gating deficit. Molecular psychiatry. 2002;7(9):1006–1011. doi: 10.1038/sj.mp.4001140. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Jockers-Scherubl MC, et al. Test-retest reliability of P50, N100 and P200 auditory sensory gating in healthy subjects. International journal of psychophysiology. 2008;67(2):81–90. doi: 10.1016/j.ijpsycho.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler L, et al. The Diagnostic Interview Schedule, Version IV. St. Louis: Washington University; 1995. [Google Scholar]
- Rosburg T, Trautner P, et al. Attention effects on sensory gating--intracranial and scalp recordings. Neuroimage. 2009;48(3):554–563. doi: 10.1016/j.neuroimage.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16(3):240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Siddle DA, Remington B, et al. Stimulus omission and dishabituation of the skin conductance response. Psychophysiology. 1983;20(2):136–145. doi: 10.1111/j.1469-8986.1983.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Sklar AL, Gilbertson R, et al. Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcoholism, clinical and experimental research. 2012;36(12):2150–2156. doi: 10.1111/j.1530-0277.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, et al. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophrenia research. 2013;147(1):196–200. doi: 10.1016/j.schres.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stevens KE, Freedman R, et al. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15(2):152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics: Second Edition. Glenview, Illinois: HarperCollins; 1989. [Google Scholar]
- Taylor B, Rehm J. The relationship between alcohol consumption and fatal motor vehicle injury: high risk at low alcohol levels. Alcoholism, clinical and experimental research s. 2012;36(10):1827–1834. doi: 10.1111/j.1530-0277.2012.01785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, et al. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res. 2011;35(6):1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim RS, Simmons AN, et al. Acute ethanol effects on brain activation in low- and high-level responders to alcohol. Alcoholism, clinical and experimental research. 2010;34(7):1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture and United States Department of Health and Human Services. Dietary guidlines for Americans, 2010. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- van Tricht MJ, Nieman DH, et al. Sensory gating in subjects at ultra high risk for developing a psychosis before and after a first psychotic episode. The world journal of biological psychiatry. 2012 doi: 10.3109/15622975.2012.680911. [DOI] [PubMed] [Google Scholar]
- Venables PH. Input Dysfunction in Schizophrenia. Progress in experimental personality research. 1964;72:1–47. [PubMed] [Google Scholar]
- Wan L, Friedman BH, et al. P50 sensory gating and attentional performance. International journal of psychophysiology. 2008;67(2):91–100. doi: 10.1016/j.ijpsycho.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE, Watson ID, et al. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. J Stud Alcohol. 1981;42(7):547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Wendt PE, Risberg J. Ethanol reduces rCFB activation of left dorsolateral prefrontal cortex during a verbal fluency task. Brain and language. 2001;77(2):197–215. doi: 10.1006/brln.2000.2434. [DOI] [PubMed] [Google Scholar]
- Yandon CA, Bugg JM, Kisley MA, Davalos DB. P50 sensory gating is related to performance on select tasks of cognitive inhibition. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(4):448–458. doi: 10.3758/CABN.9.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Zhang L, et al. Ethanol inhibition of nicotinic acetylcholine type alpha 7 receptors involves the amino-terminal domain of the receptor. Mol Pharmacol. 1996;50(4):1010–1016. [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale, Revised. Los Angeles: Western Psychological Services; 1986. [Google Scholar]