Abstract
Objectives
In the North Africa and Middle East region, the illiteracy rates among older people are high, posing a great challenge to cognitive assessment. Validated diagnostic instruments for dementia in Arabic are lacking, hampering the development of dementia research in the region. The study aimed at validating the Arabic version of the 10/66 Dementia Research Group (DRG) diagnostic assessment for dementia to determine if it is suitable for case ascertainment in epidemiological research.
Methods
244 participants older than 65 years were included, 100 with normal cognition and 144 with mild to moderate dementia. Dementia was diagnosed by clinicians according to DSM-IV criteria. Depression was diagnosed using the Geriatric Mental State. Trained interviewers blind to the cognitive status of the participants administered the 10/66 DRG diagnostic assessment to the participants and interviewed the caregivers. The discriminatory ability of the 10/66 DRG assessment and its subcomponents were evaluated against the clinical diagnoses.
Results
Half of the participants had no formal education and 49% of them were depressed. The 10/66 DRG diagnostic assessment showed excellent sensitivity (92.0%), specificity (95.1%), positive predictive value (PPV, 92.9%), and low false positive rates (FPR) among controls with no formal education (8.1%) and depression (5.6%). Each subcomponent of the 10/66 DRG diagnostic assessment independently predicted dementia diagnosis. The predictive ability of the 10/66 DRG assessment was superior to that of its subcomponents.
Conclusion
10/66 DRG diagnostic assessment for dementia is well suited for case ascertainment in epidemiological studies among Arabic speaking older population with high prevalence of illiteracy.
Keywords: Validation, Cognitive Testing, Dementia, Diagnostic Accuracy, Arabic, illiteracy
Introduction
In the North Africa and Middle East region (WHO Eastern Mediterranean Region or EMRO), due to very rapid demographic aging,1 the estimated number of people with dementia is expected to grow exponentially, 1.2 million people in 2010 rising to 2.6 million in 2030 and 6.2 million in 2050.2 This is alarming, as countries in this region lack the social and healthcare policies to meet the challenges of caring for their rapidly growing number of people with dementia. There are few population-based studies about dementia prevalence and determinants.3–6 Knowledge about societal costs, about risk and protective factors for dementia specific to the region is scarce, impeding the development of social and health policies.7
The estimated dementia prevalence of 6% among people older than 60 years in EMRO2 is based on the consensus judgment of an international expert panel8 and one good quality study from Egypt.3 Validated diagnostic instrument for dementia Arabic is lacking. Because of the high illiteracy rate among older people in the region, the commonly used Mini Mental Status Examination9 is not a suitable screening instrument because it requires arithmetical ability, reading and writing skills.10
The 10/66 Dementia Research Group (DRG) one-phase dementia diagnostic assessment has been extensively validated against clinician Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) dementia diagnosis in India, China and South East Asia, Latin America and the Spanish speaking Caribbean (Cuba, the Dominican Republic and Puerto Rico), and Africa, showing high sensitivity (94%) and specificity (94% in people with low education).11 The consistency and predictive validity of 10/66 dementia diagnosis three years after the diagnosis has also been shown.12 The 10/66 diagnostic assessment seems likely to be the most suitable for case ascertainment in a population-based study to determine dementia prevalence, incidence, risk and protective factors in Lebanon, where the illiteracy rate among people older than 65 years was 62.9% for women and 33.9% for men.13 The advantages of using the 10/66 one-phase diagnostic assessment are cost saving, simplifying statistical analysis, and reducing biases caused by a high attrition rate if a two-phase diagnostic procedure is used.11 The assessment can be administered by trained non-medical university graduates. It has been used successfully in 10/66 population-based studies to fill in the knowledge gap concerning dementia prevalence, incidence, and risk factors in many developing countries,14,15 showing that dementia occurrence in these countries is comparable or even higher than that found in developed countries,16,17 and confirming the gravity of a global epidemic.
The aim of this study is therefore to validate the 10/66 DRG diagnostic assessments in Arabic with the intention of using it in a subsequent Lebanon national population-based prevalence survey and cohort study.
Methods
Instruments
The 10/66 DRG diagnostic assessment11 is an integrated one-phase dementia diagnostic battery comprising 1) a cognitive test battery; the Community Screening Instrument for Dementia (CSI-D)18 and the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) animal naming test and modified 10 world list learning,19 2) a structured clinical interview; the Geriatric Mental State (GMS), which applies a computerized algorithm (AGECAT) to identify organic brain syndrome (dementia), schizophrenia, neurotic and psychotic depression, and anxiety neuroses,20 and 3) the CSI-D informant interview.18CSI-D combines an education and culture fair cognitive test that requires neither arithmetical ability nor reading and writing skills, and an informant interview into a predictive algorithm has been extensively validated, showing 83% specificity and 87% sensitivity.18, 21–23 10/66 dementia diagnosis, according to the initial calibration and validation, is defined as scoring above a cut-point of predicted probability for DSM-IV dementia syndrome from the logistic regression equation developed in the 10/66 international pilot study, using coefficients from the CSI-D, GMS/ AGECAT diagnostic output, and modified CERAD 10-word list learning delayed recall score.11 The overall predictive power of the algorithm exceeded that of each of the components used separately.
The CSI-D is a 32-item cognitive test for the participant covering multiple cognitive domains and a 26-item questionnaire for the caregiver inquiring about the participant’s cognitive function and functional level. The CSI-D generates three summary scores: the global cognitive score (COGSCORE), an item-weighted total score from the participant cognitive test; informant score (RELSCORE), an unweighted total score from the informant interview; and discriminant function score (DFSCORE), a weighted score combining COGSCORE and RELSCORE.18
The CERAD animal naming test requires the participants to name as many animals as they can in one minute.24 The culturally adapted CERAD ten-word list learning task19 has a learning phase during which the list is read out to the participants and they have to immediately recall the words they remember. This process is repeated three times, producing a total learning score out of 30. After 5 minutes, participants are asked to recall the ten words, producing a delayed recall score out of 10.
The GMS version B3 is a clinical interview which produces symptom scores in four diagnostic clusters (stage 1 diagnosis) from a computerized algorithm (AGECAT): organic brain syndrome (approximating to dementia), schizophrenia and related paranoid states, neurotic and psychotic depression, and anxiety neuroses. Stage 1 diagnoses are organized into final stage 2 diagnoses based on an algorithm that is hierarchically structured.21,25
Translation
Two research assistants who are Master of Public Heath (MPH) graduates with proficiency in both Arabic and English independently translated the 10/66 diagnostic assessment. Subsequently, meetings were held with community elderly representatives, health professionals and interviewers, to discuss the conceptual validity of the questionnaires in the Lebanese cultural and linguistic contexts. The translated diagnostic assessment was then pilot tested under field conditions in 10 individuals to assess the interview burden and the acceptability of the questionnaires.
A few items in the CSI-D were modified. First, in the body-part-naming test, “knuckle” was substituted by “chin”, since there is no word for knuckle in Arabic. Second, the phrase testing for verbal fluency “no ifs, ands, or buts” was replaced with a popular tongue twister in Arabic “خيط حرير على حيط أم خليل”, translated as “Silk string on Um Khalil’s wall”. Third, the common knowledge question “what is the name of mayor/village head” was changed to “what is the name of the current president.” Fourth, the long-term memory question was substituted with the local equivalence of: “Who was the president-elect that was assassinated after three weeks in office in 1982 during the first Israelis invasion of Lebanon?” The GMS and CSI-D informant interview were contextually translated without need for modification.
Training
The leader of the 10/66 DRG (MP) trained the study coordinator (KP) during one week in the study protocol, data handling, data entry, and 10/66 assessment. It was followed by a three-day training program for the Lebanese research team, consisting of an epidemiologist (MC), a neurologist (SA), an old-age psychiatrist (GK), a geriatrician (HG), a research assistant, and six interviewers. Interviewers were clinicians (neurological and geriatric fellows, and family medicine resident), and non-medical university graduate (MPH). The training for the interviewers in administering the GMS took one full day; during with they viewed and co-rated two training tapes with MP and GK. Subsequently, the interviewers were trained on 10 live volunteers under field conditions. The quality control section below provides more information about the training.
Participants
For the estimation of a proportion of 92% - midway between the sensitivity of 94% and specificity of 90% estimated in 10/66 first pilot study,11 the minimum required sample size to achieve a maximal error of ±5% is 200 persons, equally divided between participants with normal cognition and dementia. Participants were recruited from social organizations for the elderly, hospital-based neurological and geriatric clinics, and community-based primary care clinics. Inclusion criteria were 1) age 65 years and older, 2) having normal cognition (controls) or with mild to moderate dementia (cases), and 3) having an informant who could give an independent assessment for the older participant’s health and cognitive status. Exclusion criteria were 1) severe somatic or psychiatric illness when the diagnosis of dementia could not be ascertained without extensive medical work-up and neuropsychological testing, and 2) mild cognitive impairment (MCI) diagnosed according to the core clinical criteria for MCI by the National Institute on Aging - Alzheimer’s Association Workgroups.26
Dementia diagnoses were established by clinicians according to DSM-IV criteria.27 Dementia severity was rated using Clinical Dementia Rating scale (CDR).28 Depression was diagnosed using the GMS/AGECAT stage 1 output, which has a sensitivity of 90% for diagnosing depression among older people in developing countries.29
The participating organizations and clinics identified and referred potential cases and controls. In the hospital-based geriatric and neurology clinics, dementia and MCI diagnoses were established by geriatricians and neurologists. The study coordinator reviewed the case notes and discussed with the specialists to confirm the cognitive status and to include and exclude participants according to the predefined criteria. When the clinical information from the case notes was insufficient, the clinicians from the research team, in consensus with the specialists from the clinics, carried out supplementary cognitive assessment of the participants and interviewed the caregivers. A case finding approach was used in the social organizations and community-based primary care clinics, where the staff of the organizations was asked to identify and nominate participants with cognitive complaints. Clinicians from the research team carried out a cognitive assessment for the nominated participants and interviewed their caregivers to diagnose dementia and MCI according to the research criteria mentioned above.
The interviewers blind to the cognitive status of the participants administered the CSI-D and the GMS to the participants and the CSI-D informant to their caregivers. Data collection was completed from March 2012 to February 2013.
Quality Control
The study coordinator (KP) along with the Arabic speaking research assistant (RMK) accompanied each of the newly-trained interviewers as they conducted the field work until they could conduct the interview in a standardized manner. All interviewers’ scripts were reviewed by the study coordinator and the research assistant. Weekly meetings with all interviewers were held to discuss the problems they encountered during the field work and to correct the errors identified during the script review. Double data entry was done to minimize errors.
Ethics
The Institutional Review Board of the American University of Beirut, Lebanon approved the study. All participants gave written informed consent, or relatives gave consent on behalf of participants with impaired decision making capacity. Decision making capacity was assessed in all participants with dementia through a checklist to ensure their complete comprehension about the nature of the study and the protection of their right.
Statistical analysis
The following parameters were estimated for all subcomponents of the 10/66 DRG diagnostic assessment (CSI-D COGSCORE and DFSCORE, GMS/AGECAT, CERAD 10 word list learning test) and the 10/66 dementia diagnostic algorithm against clinician dementia diagnosis: Sensitivity, specificity, positive predictive value (PPV), false positive rate (FPR) among participants with depression according to stage 1 GMS/AGECAT output, and FPR among people with no formal education. The no formal education group included people who were illiterate or could barely read and write. The formal education group included those with primary education and above. The previously derived item weights and cut-points for possible and probable cases for CSI-D algorithm were used.18,22 Dementia diagnosed by GMS/AGECAT was stage 2 output of organic cases. For 10/66 DRG diagnostic algorithm, the optimal cut-point probability derived from the logistic regression model in the calibration phase of the original cross-cultural development and validation was used.11 The Youden index [(sensitivity + specificity)−1] was used to summarize sensitivity and specificity in a single measure at the chosen cut-points for 10/66 DRG dementia diagnosis, DFSCORE and COGSCORE, GMS/AGECAT stage 2 output for dementia, and cut-point with the best sensitivity and specificity for CERAD 10 word delayed recall found in this study (see below). The overall predictive ability of the 10/66 DRG diagnostic assessment and its sub-components was evaluated as the area under the receiver-operator characteristics curve (AUROC: sensitivity plotted against 1- specificity), using the full range of ordinal scores for the predicted probability of 10/66 DRG assessment, raw scores for the CERAD 10 word list delayed recall, CSI-D DFSCORE and COGSCORE. The AUROC was used to find the cut-point with the best sensitivity and specificity for CERAD 10 word list delayed recall.
The 10/66 DRG assessment was designed to diagnose people with dementia living in the community, where the informant is typically a non-medical relative or a friend. However, half of the cases in our study sample resided in long term care institutions, where the informant was a nurse or a physiotherapist. Also, the older participants residing in long term care institutions were frailer than those living in the community, a factor that might limit their test performance. Therefore, we carried out a sensitivity analysis stratified by type of residence to assess the performance of the instrument in these two different settings.
Results
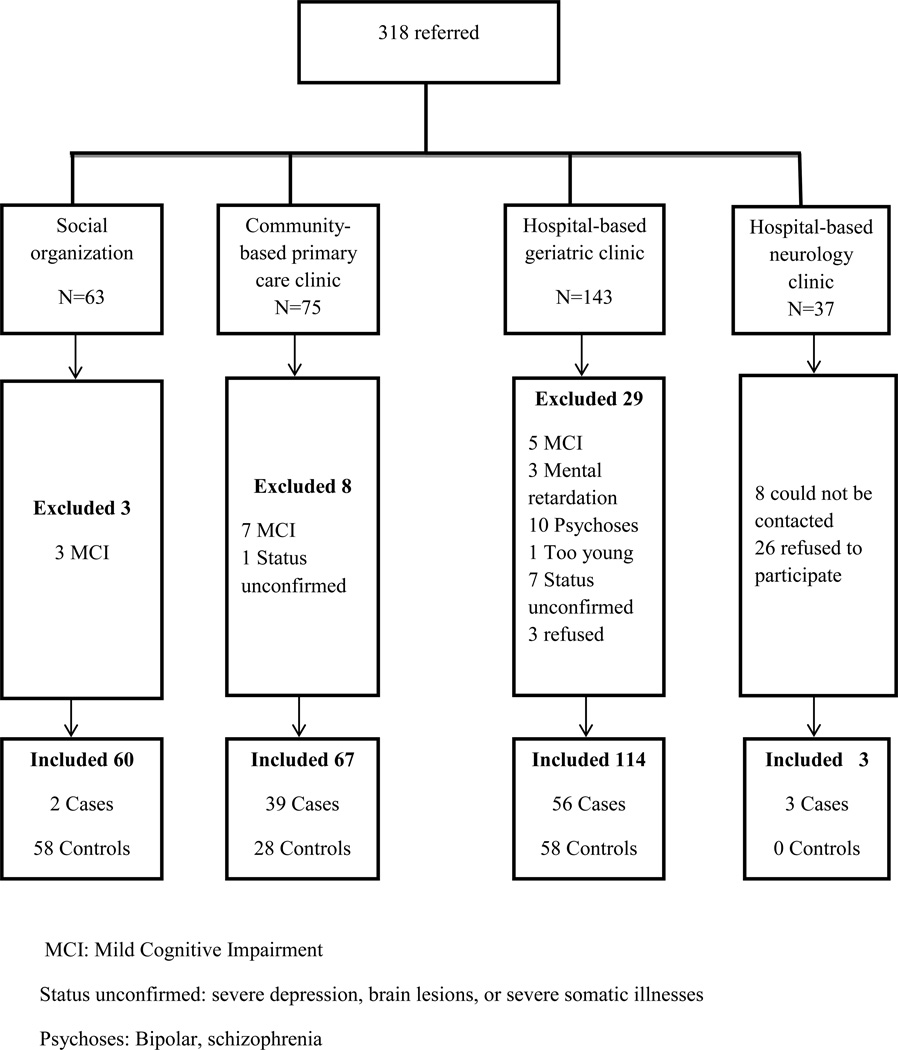
The social organizations and clinics referred 318 persons older than 65. We excluded 40 persons (Figure 1). Eight persons could not be contacted and 29 refused to participate. The overall response rate was 88.4%. Because those who refused to participate or did not respond were mostly from the hospital-based private neurology clinics, where patients pay high fees for the consultations, they most likely belonged to a socioeconomically advantaged group (Figure 1). The demographic characteristics of the 144 controls and 100 cases are presented in Table 1. Most participants were recruited from the social organizations, community-based primary care clinics, and hospital-based geriatric clinics, which are mainly charity organizations. Therefore, most participants had low socioeconomic status and the prevalence of people with no formal education in the study sample was high (50.4%), 18.4% had completed primary education, 13.1% intermediate, 11.1% secondary school, and 7.0% university and above (Table 1). 27.5% of those lacking formal education were illiterate. In all, 114 persons referred from hospital-based geriatric clinics resided in long term care institutions. From here, we excluded 24 persons: ten with psychoses, two with brain lesions, three with MCI, three with mental retardation, five with severe depression or somatic illness, and one too young. As a result, 41 controls and 49 cases from long term care institutions were included (Table 1). Compared with the long term care home residents, community-dwelling participants were younger, with more controls and more cases of mild dementia. The participants came from urban and rural areas across the three representative geographical regions of Lebanon; 54.1% from the coastal strip (Beirut), 21.3% from the mountain towns and villages (Shouf and Dhour Shweir), and 24.6% from the central highland villages (the Bekaa valley).
Figure 1.
Recruitment flowchart
Table 1.
Demographic characteristics of the study population
| Residence | Community | Institution | Total (%) | ||
|---|---|---|---|---|---|
| Cognitive Status | Dementia | Control | Dementia | Control | |
| Total (N) | 51 | 103 | 49 | 41 | 244 |
| 144 (59.0) controls | |||||
| 31 mild | 22 mild | 53 (21.7) mild dementia | |||
| 20 moderate | 27 moderate | 47 (19.3) moderate dementia | |||
| Age | |||||
| 65–74 years | 8 | 53 | 7 | 10 | 78 (31.9) |
| 75–84 years | 30 | 35 | 19 | 15 | 99 (40.5) |
| 85–100 years | 13 | 15 | 23 | 16 | 67 (27.5) |
| Gender | |||||
| Female | 32 | 70 | 37 | 19 | 158 (64.8) |
| Male | 19 | 33 | 12 | 22 | 86 (35.2) |
| Education | |||||
| No formal education | 33 | 48 | 28 | 14 | 123 (50.4) |
| Primary (grade 5) | 11 | 19 | 7 | 8 | 45 (18.4) |
| Intermediate (grade 9) | 5 | 15 | 6 | 6 | 32 (13.1) |
| Secondary (grade 12) | 2 | 10 | 6 | 9 | 27 (11.1) |
| University and above | 0 | 11 | 2 | 4 | 17(7.0) |
| Recruitment Site | |||||
| Hospital-based neurology clinics | 3 | 3 (1.3) | |||
| Hospital-based geriatric clinics | 7 | 17 | 49 | 41 | 114 (59.0) |
| Community-based primary care clinics | 39 | 28 | 67 (27.5) | ||
| Social organizations | 2 | 58 | 60 (24.6) | ||
Forty-nine (49.0%) participants with dementia had co-morbid depression, and 71 (49.3%) controls were depressed. The 10/66 DRG algorithm yielded 99 dementia cases; the GMS/AGECAT output 125 dementia cases (organic cases); the CSI-D DFSCORE 106 dementia cases (18 possible and 88 probable); and CSI-D COGSCORE 171 dementia cases (28 possible and 143 probable). The psychometric properties of 10/66 DRG diagnostic assessment and its sub-components are reported in Table 2. The 10/66 DRG diagnostic assessment showed excellent sensitivity and specificity, and low FPR among controls with no formal education and controls with depression (Table 2). Its discriminatory ability was superior to that of its sub-components.
Table 2.
Psychometric properties of 10/66 DRG diagnostic assessment and its subcomponents
| Sensitivity | Specificity | PPV | Youden’s index |
Area under ROC curve |
Depression FPR |
No formal education FPR |
Formal education FPR |
|
|---|---|---|---|---|---|---|---|---|
| % (95% CI) |
% (95% CI) |
% (95% CI) |
(95% CI) | % (95% CI) |
% (95% CI) |
% (95% CI) |
||
| N=244 | N=244 | N=244 | N=244 | N=244 | N=120 | N=123 | N= 121 | |
| 10/66 DRG | ||||||||
| Diagnostic Assessment | 92.0 (85.0,95.9) | 95.1 (90.3,97.6) | 92.9 (86.1,96.5) | 0.87 | 0.97 (0.95,0.99) | 5.6 (2.2,13.6) | 8.1 (3.5,17.5) | 2.4 (0.7,8.5) |
| GMS | 93.0 (86.3,96.6) | 77.8 (70.3,83.8) | 74.0 (66.1,81.2) | 0.71 | N/A | 26.8 (17.9,38.1) | 37.1 (26.2,49.5) | 11.0 (5.9,19.6) |
| CERAD 10 word list delayed recall | 91.0 (83.8,95.2) | 67.4 (59.3,74.5) | 65.9 (57.7,73.3) | 0.58 | 0.87 (0.82,0.91) | 85.9 (76.0,92.2) | 33.9 (23.3,46.3) | 31.7 (22.7,42.4) |
| CSI-D DFSCORE | 92.0 (85.0,95.9) | 90.3 (83.3,94.1) | 86.8 (79.0,92.0) | 0.82 | 0.97 (0.96,0.99) | 12.7 (6.8,22.4) | 9.7 (4.5,19.6) | 9.8 (5.0,18.1) |
| CSI-D COGSCORE | 98.0 (93.0,99.5) | 49.3 (41.3,57.4) | 57.3 (49.8,64.5) | 0.47 | 0.95 (0.92,0.97) | 59.2 (47.5,69.8) | 67.7 (55.4,78.1) | 37.8 (28.1,48.6) |
CI: Confidence Interval; ROC: Receiver-Operator Characteristics; PPV: Positive Predictive Value; FPR: False Positive Rate; DRG: Dementia Research Group; GMS: Geriatric Mental State; CERAD: Consortium to Establish a Registry of Alzheimer’s Disease; CSID: Community Screening Instrument for Dementia, N/A: Not applicable
CSI-D demonstrated excellent sensitivity (92.0%) and specificity (90.3%, Table 2). CSI-D had difficulty distinguishing dementia from depression with a 12.7% FPR. The FPR was similar among controls with and without formal education (9.8% and 9.7%, respectively; Table 2). CSI-D DFSCORE combining CSI-D COGSCORE and RELSCORE was better than COGSCORE alone, as sensitivity was slightly reduced, but specificity was markedly improved, and the FPR was markedly reduced among controls who were depressed and with no formal education (Table 2). CSI-D COGSCORE has very high sensitivity (98.0%) but remarkably low specificity in our study (49.3%).
Using the AUROC curve, we found that the cut-point of three for CERAD 10 word list delayed recall yielded the best sensitivity and specificity of 91.0% and 67.4%, respectively (Table 2). There was a high FPR among both people with and without formal education (Table 2). It performed very poorly among people with depression with a FPR of 85.9%.
The GMS had excellent sensitivity (94.0%) but unsatisfactory specificity (77.1%), with high FPR among controls with no formal education (37.1%) and to a lesser extent controls who were depressed (26.8%).
The sensitivity analysis stratified by places of residence showed that the 10/66 DRG diagnostic assessment had comparable sensitivity and specificity to detect dementia among people residing in the community and in the institutions (Table 3). Because the prevalence of dementia among participants residing in the institutions (49.0%) was higher than the prevalence of dementia among participants living in the community (33.1%, Table 1), the PPV of the 10/66 DRG assessment was better when used in the institutional setting (Table 3).
Table 3.
Sensitivity and specificity stratified by type of residence
| Type of Residence | Sensitivity % (95% CI) |
Specificity % (95% CI) |
PPV % (95% CI) |
|---|---|---|---|
| Community | |||
| N=154 | |||
| 10/66 DRG Diagnostic Instrument | 92.2 (81.5,96.9) | 93.2 (86.6,96.7) | 87.0 (75.6,93.6) |
| GMS | 94.1 (84.1,98.0) | 78.6 (69.8. 85.4) | 68.6 (57.0, 78.2) |
| CSI-D DFSCORE | 88.2 (76.6,94.5) | 91.3 (84.2,95.3) | 83.3 (71.3,91.0) |
| CSI-D COGSCORE | 98.0 (89.7,99.7) | 58.3 (48.6,67.3) | 53.8 (43.7,63.6) |
| Institution | |||
| N= 90 | |||
| 10/66 DRG Diagnostic Instrument | 91.8 (80.8,96.8) | 100 (91.4, 100) | 100 (92.1,100) |
| GMS | 93.9 (83.5,97.9) | 73.2 (58.1,84.3) | 80.7 (68.7,88.9) |
| CSI-D DFSCORE | 95.9 (86.3,98.9) | 87.8 (74.5,94.7) | 90.4 (79.4,95.8) |
| CSI-D COGSCORE | 98.0 (89.3,99.6) | 26.8 (15.7,41.9) | 61.5 (50.4,71.6) |
PPV: Positive Predictive Value; CI: Confidence Interval; DRG: Dementia Research Group; GMS: Geriatric Mental State; CSI-D: Community Screening Instrument for Dementia
Discussion
This study showed that the 10/66 diagnostic assessment has excellent discriminatory ability to diagnose dementia in an Arabic speaking older population with a high prevalence of illiteracy. It is therefore a valid diagnostic assessment for a subsequent population-based study about dementia prevalence, incidence, and risk factors in Lebanon.
The strength of our study was strictly standardizing the procedures according to 10/66 DRG protocol through rigorous translation process, training and supervision of interviewers, and case ascertainment. Case ascertainment was done by clinicians from teaching hospitals in Lebanon. Due to lack of resources, there were not sufficient paraclinical investigations (laboratory tests and neuroimaging) to determine the underlying causes of dementia in most cases. Since neuropsychological testing and paraclinical investigations for the MCI cases were not available, we could have excluded some very mild dementia cases from the study. The case-finding approach used in the social organizations and community-based primary care clinics could lead to a misclassification of participants with mild dementia as controls, since the staff in these organizations could have missed cognitive symptoms in some participants and did not nominate them for clinical assessment. This non-differential misclassification could underestimate the discriminatory ability of the 10/66 DRG diagnostic assessment. Conversely, the inclusion of participants from the long term care setting, where the prevalence of dementia is high (60%)30 and informants are health care professionals, could lead to an overestimation of the assessment’ discriminatory ability. However, because we recruited both cases and controls from the hospital-based long term care units, we were able to show the robustness of the methodology across community and long term care settings.
In the previous multicenter 10/66 DRG validation study, education was stratified into low education group (80–90% with no or minimal education) and high education (80–99% with secondary education).11 The FPR of CSI-D among people with high education in the previous multicenter 10/66 DRG validation study was only 4%.11 Since this study focused on the performance of the 10/66 DRG diagnostic assessment among Arabic speaking older people with no formal education, education was stratified into two groups: no formal education (100% with no or minimal education) and formal education (only 36.4% with more than secondary education). The study sample was small therefore the formal education group was not stratified further. This could explain why the CSI-D and CERAD 10 word list delayed recall had high FPR for people with and without formal education in this study.
Prince et al has shown that GMS over-diagnosed dementia among people with low education.11 This educational bias was corrected by adding CSI-D to the 10/66 DRG algorithm. GMS is a key element of 10/66 assessment because of its ability to reduce the FPR among people with depression, but GMS is not free from the bias caused by depression. The high sensitivity of the GMS in this study could be because two-thirds of our interviewers are neurological and geriatric fellows who are well-trained in diagnosing dementia. Although they were trained to code the GMS objectively, their clinical judgment was taken into account in several items that are incorporated into GMS/AGECAT output. The most effective discriminative item of the GMS has been shown to be the interviewer’s global assessment of the cognitive status.29
Consistent with results from the previous multicenter 10/66 DRG validation study, this study showed that the 10/66 DRG diagnostic algorithm had superior specificity and PPV compared to all three sub-components, and sensitivity was only slightly compromised. This assessment was validated to be used it in a longitudinal cohort study about dementia prevalence, incidence, and risk factors in Lebanon. Prince et al has estimated a dementia prevalence of 6% among the older population in the Middle East,2 a much lower prevalence than the 41.0% prevalence in this selected study sample of controls and patients with dementia. Therefore, a diagnostic instrument with high PPV is best suited for the population-based setting. The 10/66 one-phase diagnostic assessment, which integrates all three cognitive measures (CSI-D, CERAD, and GMS) with the best specificity (95.1%) and PPV (92.9%), and excellent sensitivity (92.0%), can best serve this purpose.
The sensitivity analysis stratified by type of residence to evaluate the performance of the 10/66 DRG assessment in the community and institutions showed that the sensitivity and specificity were comparable in these two settings. The slightly higher specificity in the institution setting could reflect that fact that the medical staff in the institutions had better knowledge of the participants’ cognitive status. Their input well compensated for the very poor specificity of the CSI-D COGSCORE, which could be caused by the frailty and physical disabilities of people residing in institutions. This analysis confirmed the excellent performance of the 10/66 DRG diagnostic assessment in a community setting.
Acknowledgement
The study was funded by the Fogarty International Center, American National Institute of Health and National Institute of Aging, grant number 1R21AG039333-01 under the program “Brain Disorders in the Developing World: Research Across Lifespan (BRAIN)”. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The study is collaboration between the Department of Epidemiology and Population Health, Faculty of Health Sciences, and the Department of Neurology from the American University of Beirut, Lebanon; the Danish Dementia Research Center, Department of Neurology, Rigshospitalet, University of Copenhagen, Denmark; and the Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, London, UK.
The recruitment was a community effort with contribution from institutions, organizations, and individuals. Above all, the research group would like to express our deep gratitude to the participants in the study. We would also like to thank the Ministry of Social Affairs’ Social Center in Bourj Hammoud; The NGOs Restaurant du Coeur, the ladies from the Board of the Child & Mother Welfare Society, and Ayadina Social Center for the Elderly; Ain W Zein Hospital Geriatric Clinic; Neurology Clinic at AUB Medical Center; geriatric clinics from Al Saydeh Hospital in Antelias, Child & Mother Welfare Society, and Baytouna Nursing Home; primary care clinics from Maronite Nursing Home, Al Saydeh Church in Hamra, and Al Salib Church in Naaba; social workers Glady Farrah and Mona Saliby; Mrs. Dora Chaaya; Dean Iman Nuwayhid and Professor Jihad Makhoul from the Faculty of Health Sciences, AUB; and Mrs. Helen Samaha, President of the Lebanese Order of Nursing. Dr. Ali Al Ahmar, Dr. Wael Rahwan, Dr. Salem Hammoud, Dr. Sandrine Salman, Dr. Ibrahim Zeinaty, and Ms. Rose Mary Khoury worked as interviewers in the study.
We would like to thank Alzheimer Association Lebanon for providing the educational materials about dementia for the patients and their caregivers in this study.
Footnotes
Data sharing: The Arabic version of the 10/66 DRG diagnostic instrument is available upon request either to Dr. Kieu Phung at thien.phungmail.mcgill.ca or Professor Monique Chaaya at mchaayaaub.edu.lb. It will be available for downloading from the 10/66 DRG website at http://www.alz.co.uk/1066/.
For the purpose of universal use in all Arabic speaking countries, the translation is in classical Arabic. The interviewers were trained in asking the questions in the spoken Arabic specific to Lebanon in a standardized manner.
Requests to gain access to data can be directed to Professor Monique Chaaya at mchaayaub.edu.lb.
References
- 1.Yount KM, Sibai AM. Demography of ageing in Arab countries. In: Unlengerg P, editor. International Handbook of Population Aging. Springer Science + Business Media B.V.; 2009. pp. 277–314. [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Farrag A, Farwiz HM, Khedr EH, et al. Prevalence of Alzheimer's disease and other dementing disorders: Assiut-Upper Egypt study. Dement. Geriatr. Cogn Disord. 1998;9:323–328. doi: 10.1159/000017084. [DOI] [PubMed] [Google Scholar]
- 4.Bowirrat A, Treves TA, Friedland RP, et al. Prevalence of Alzheimer's type dementia in an elderly Arab population. Eur J Neurol. 2001;8:119–123. doi: 10.1046/j.1468-1331.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 5.Ghubash R, El-Rufaie O, Zoubeidi T, et al. Profile of mental disorders among the elderly United Arab Emirates population: sociodemographic correlates. Int J Geriatr Psychiatry. 2004;19:344–351. doi: 10.1002/gps.1101. [DOI] [PubMed] [Google Scholar]
- 6.Afgin AE, Massarwa M, Schechtman E, et al. High prevalence of mild cognitive impairment and Alzheimer's disease in Arabic villages in northern Israel: impact of gender and education. J Alzheimers Dis. 2012;29(2):431–439. doi: 10.3233/JAD-2011-111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince M. Dementia in developing countries. A consensus statement from the 10/66 Dementia Research Group. Int. J. Geriatr. Psychiatry. 2000;15:14–20. doi: 10.1002/(sici)1099-1166(200001)15:1<14::aid-gps70>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Scazufca M, Almeida OP, Vallada HP, et al. Limitations of the Mini-Mental State Examination for screening dementia in a community with low socioeconomic status:Results from the Sao Paulo Ageing & Health Study. Eur Arch Psychiatry Clin Neurosci. 2009;259:8–15. doi: 10.1007/s00406-008-0827-6. [DOI] [PubMed] [Google Scholar]
- 11.Prince M, Acosta D, Chiu H, et al. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 12.Jotheeswaran AT, Williams JD, Prince MJ. The predictive validity of the 10/66 Dementia diagnosis in Chennai, India – a three year follow-up study of cases identified at baseline. Alzheimer Dis Assoc Disord. 2010;24:296–302. doi: 10.1097/WAD.0b013e3181d5e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibai AM, Sen K, Baydoun M, Saxena P. Population ageing in Lebanon: current status, future prospects and implications for policy. Bull. World Health Organ. 2004;82:219–225. [PMC free article] [PubMed] [Google Scholar]
- 14.Prince M, Acosta D, Ferri C, et al. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. The Lancet. 2012;380:50–58. doi: 10.1016/S0140-6736(12)60399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez J, Ferri C, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. The Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman A, Rocca WA, Brayne C, et al. The prevalence of dementia in Europe: a collaborative study of 1980–1990 findings. Eurodem Prevalence Research Group. Int J Epidemiol. 1991;20:736–748. doi: 10.1093/ije/20.3.736. [DOI] [PubMed] [Google Scholar]
- 17.Launer LJ, Andersen K, Dewey ME, et al. Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 18.Hall KS, Hendrie HH, Brittain HM, et al. The development of a dementia screening interview in two distinct languages. Int J Methods Psychiatric Res. 1993;3:1–28. [Google Scholar]
- 19.Ganguli M, Chandra V, Gilby JE, et al. Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-U.S. Cross-National Dementia Epidemiology Study. Int. Psychogeriatr. 1996;8:507–524. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- 20.Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol. Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 21.Hall KS, Gao S, Emsley CL, et al. Community screening interview for dementia (CSI 'D'); performance in five disparate study sites. Int. J. Geriatr. Psychiatry. 2000;15:521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Hendrie HC, Hall KS, Pillay N, et al. Alzheimer's disease is rare in Cree. Int. Psychogeriatr. 1993;5:5–14. doi: 10.1017/s1041610293001358. [DOI] [PubMed] [Google Scholar]
- 23.Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am. J. Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 24.Goodglass H, Kaplan E. Assessment of dysphasia and related disorders. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 25.Copeland JR, Dewey ME, Saunders P. The epidemiology of dementia: GMS-AGECAT studies of prevalence and incidence, including studies in progress. European Archives of Psychiatry and Clinical Neuroscience. 1991;240:212–217. doi: 10.1007/BF02189529. [DOI] [PubMed] [Google Scholar]
- 26.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 29.Prince M, Daisy A, Chiu H, et al. Effects of education and culture on the validity of the Geriatric Mental State and its AGECAT algorithm. British Journal of Psychiatry. 2004;185:429–436. doi: 10.1192/bjp.185.5.429. [DOI] [PubMed] [Google Scholar]
- 30.Chahine LM, Bijlsma A, Hospers AP, Chemali Z. Dementia and depression among nursing home residents in Lebanon: a pilot study. Int. J. Geriatr. Psychiatry. 2007;22:283–5. doi: 10.1002/gps.1663. [DOI] [PubMed] [Google Scholar]