Abstract
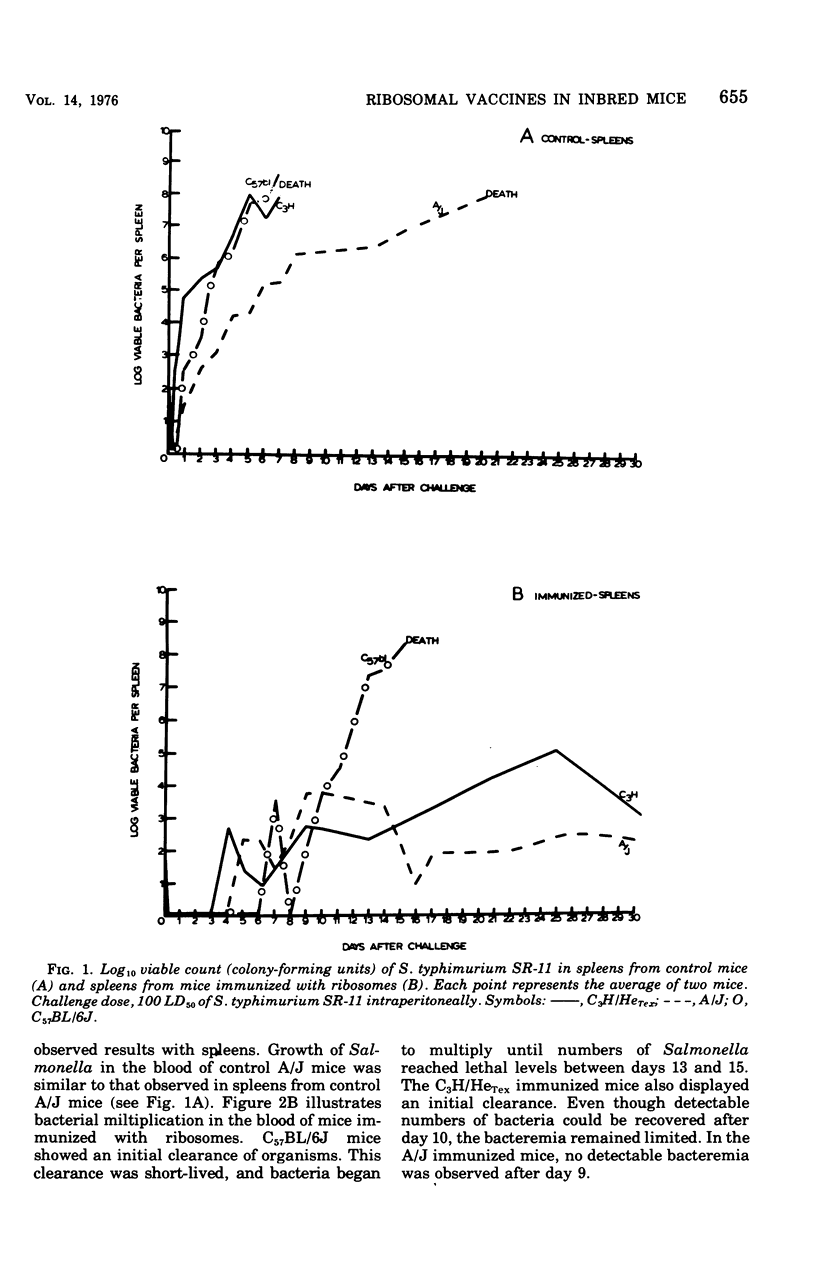
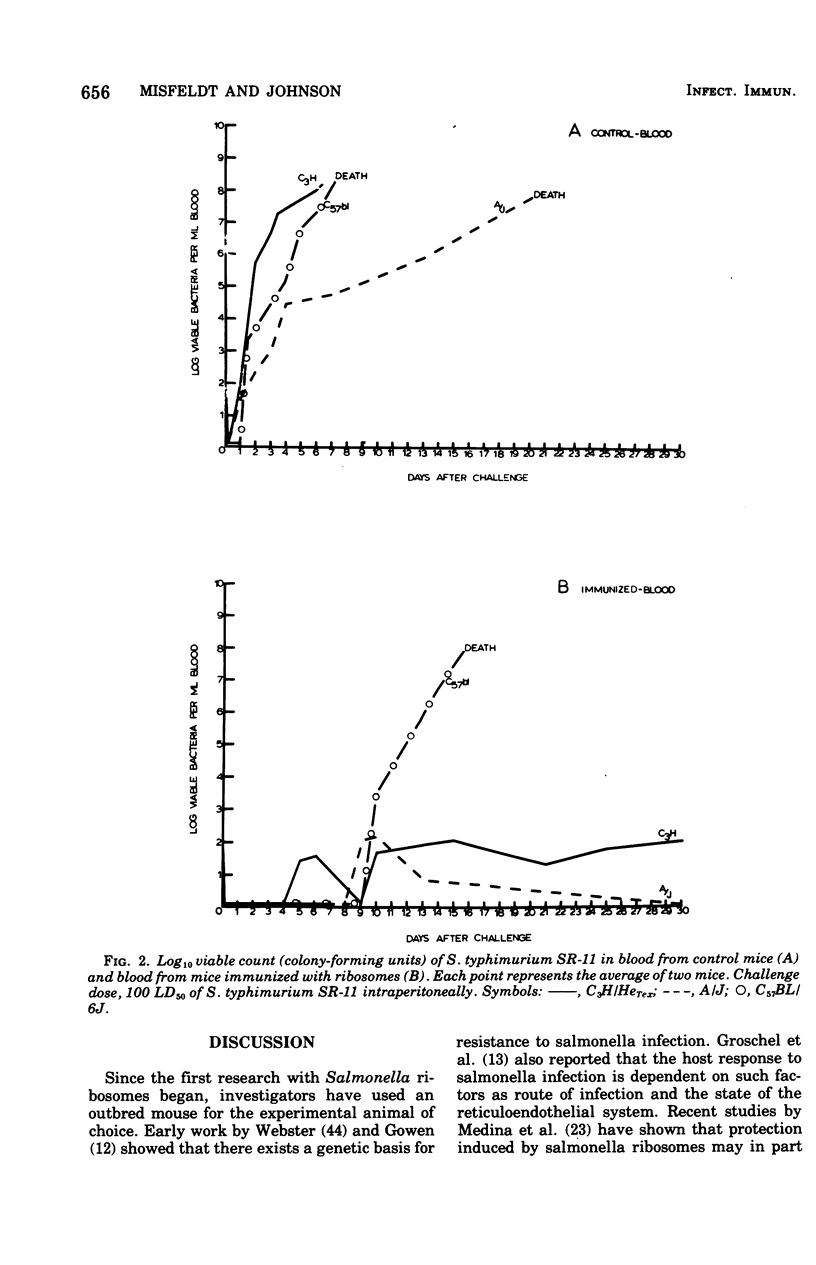
Ribosomal vaccines prepared from Salmonella typhimurium were effective immunogens in A/J inbred mice and C3H/HeTex, inbred mice. However, ribosomal vaccines were not protective in C57BL/6J inbred mice. A/J mice were protected against lethal challenge by attenuated S. typhimurium live-cell, ribosomal, phenol, and heat-killed vaccines. C3H/HeTex mice were protected by live-cell, ribosomal, and phenol vaccines but not the heat-killed vaccine. Only the live-cell vaccine gave significant protection in the C57BL/6J inbred mice. A comparison of the kinetics of infection in sham-immunized mice and mice immunized with ribosomes showed that ribosome preparations elicited protection against Salmonella infection in mice inherently sensitive and resistant to Salmonella.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andron LA I. I., Eigelsbach H. T. Biochemical and immunological properties of ribonucleic acid-rich extracts from Francisella tularensis. Infect Immun. 1975 Jul;12(1):137–142. doi: 10.1128/iai.12.1.137-142.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 2. Further evidence for varying susceptibility of outbred mice and evaluation of the response of 5 inbred mouse strains to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1974 Jul;82(4):459–474. [PubMed] [Google Scholar]
- Collins F. M., Carter P. B. Comparative immunogenicity of heat-killed and living oral Salmonella vaccines. Infect Immun. 1972 Oct;6(4):451–458. doi: 10.1128/iai.6.4.451-458.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel M. J. The immunogenic activity of ribosomal fractions derived from Brucella abortus. J Hyg (Lond) 1976 Feb;76(1):65–74. doi: 10.1017/s0022172400054954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit C., Tewari R. P. Immunogenicity of Ribosomal Preparations from Yeast Cells of Histoplasma capsulatum. Infect Immun. 1974 Nov;10(5):1091–1097. doi: 10.1128/iai.10.5.1091-1097.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOWEN J. W. Genetic effects in nonspecific resistance to infectious disease. Bacteriol Rev. 1960 Mar;24(1):192–200. doi: 10.1128/br.24.1.192-200.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschel D., Paas C. M., Rosenberg B. S. Inherited resistance and mouse typhoid. I. Some factors which affect the survival of infected mice. J Reticuloendothel Soc. 1970 Apr;7(4):484–499. [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Protection of suckling mice from experimental cholera by maternal immunization: comparison of the efficacy of whole-cell, ribosomal-derived, and enterotoxin immunogens. Infect Immun. 1974 Jul;10(1):167–172. doi: 10.1128/iai.10.1.167-172.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G. Resistance to infection with Salmonella paratyphi C in mice parasitized with a relatively avirulent strain of Salmonella typhimurium. Nature. 1961 Jul 1;191:87–88. doi: 10.1038/191087a0. [DOI] [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchens D. P., Wright G. L., Jr Immunity to Salmonella typhimurium infection: characterization of antigens in active protection by polyacrylamide gel electrophoresis. Infect Immun. 1973 Mar;7(3):507–511. doi: 10.1128/iai.7.3.507-511.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Gregory B., Naylor J., Actor P. Isolation of protective somatic antigen from Vibrio cholerae (Ogawa) ribosomal preparations. Infect Immun. 1972 Aug;6(2):156–161. doi: 10.1128/iai.6.2.156-161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. doi: 10.1128/iai.5.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- Nomoto K., Makidono R., Takeya K. Immune response against hamster erythrocytes in the low-responder mouse strains. IV. Delayed hypersensitivity against solubilized hamster erythrocytes in mice. Jpn J Microbiol. 1972 Sep;16(5):415–423. doi: 10.1111/j.1348-0421.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- Portelance V., Brasseur R., Boulanger R. P. Factors affecting the immunizing activity of ribosomal fractions isolated from Mycobacterium tuberculosis var. bovis strain BCG. Can J Microbiol. 1975 Oct;21(10):1492–1499. doi: 10.1139/m75-221. [DOI] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Schalla W. O., Johnson W. Immunogenicity of ribosomal vaccines isolated from group A, type 14 Streptococcus pyogenes. Infect Immun. 1975 Jun;11(6):1195–1202. doi: 10.1128/iai.11.6.1195-1202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Wysocki J. A., Bruun J. N., De Courcy S. J., Jr, Blakemore W. S., Mudd S. Efficacy of ribosomal preparations from Pseudomonas aeruginosa to protect against intravenous Pseudomonas challenge in mice. J Reticuloendothel Soc. 1974 Jan;15(1):22–30. [PubMed] [Google Scholar]
- Thiele E. H. Induction of host resistance in different mouse strains. Proc Soc Exp Biol Med. 1974 Sep;146(4):1067–1070. doi: 10.3181/00379727-146-38247. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Weiss E. Response of mice to injection of ribosomal fraction from group B Neisseria meningitidis. Infect Immun. 1972 Sep;6(3):355–363. doi: 10.1128/iai.6.3.355-363.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Eisenstein T. K. Biological properties of an immunogenic pneumococcal subcellular preparation. Infect Immun. 1976 Mar;13(3):750–757. doi: 10.1128/iai.13.3.750-757.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S. H., Berry L. J. Antibacterial immunity induced by ribosomal vaccines. J Reticuloendothel Soc. 1970 Jul;8(1):13–24. [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. NATURE OF THE LABILE IMMUNOGENIC SUBSTANCE IN THE PARTICULATE FRACTION ISOLATED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Oct;88:1030–1037. doi: 10.1128/jb.88.4.1030-1037.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Failure of synthetic polynucleotides to affect the immunogenicity of mycobacterial ribonucleic Acid and ribosomal protein preparations. Infect Immun. 1971 Jan;3(1):149–153. doi: 10.1128/iai.3.1.149-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


