Abstract
During the last two decades, various local thermal ablative techniques for the treatment of unresectable hepatocellular carcinoma (HCC) have been developed. According to internationally endorsed guidelines, percutaneous thermal ablation is the mainstay of treatment in patients with small HCC who are not candidates for surgical resection or transplantation. Laser ablation (LA) represents one of currently available loco-ablative techniques. In this article, the general principles, technique, image guidance, and patient selection are reported. Primary effectiveness, long-term outcome, and complications are also discussed. A review of published data suggests that LA is equivalent to the more popular and widespread radiofrequency ablation in both local tumor control and long-term outcome in the percutaneous treatment of early HCC. In addition, the LA technique using multiple thin laser fibres allows improved ablative effectiveness in HCCs greater than 3 cm. Reference centres should be equipped with all the available techniques so as to be able to use the best and the most suitable procedure for each type of lesion for each patient.
Keywords: Liver, Hepatocellular carcinoma, Minimally invasive procedures, Laser, Laser ablation
Core tip: The aim of this review is to describe the basic principles, results in terms of safety and efficacy, and recent advancements in laser ablation (LA). This mini-invasive technique is a less known and few employed procedure as compared to radiofrequency ablation (RFA). However, according to published studies LA is as safe and effective as RFA. In the review the technique and potential advantages of LA are described. Our ambition is to provide the hepatologists, and other physicians, with an updated approach to this ablative technique.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a global health problem, ranking as the sixth most common malignancy and the third most frequent cause of cancer-related death worldwide[1-3]. Its incidence is rising, mostly due to the diffusion of hepatitis B or C virus infection, alcohol-related cirrhosis, and nonalcoholic steatohepatitis[2,4]. Its incidence increases with advancing age and is more common in males[5,6]. Thanks to semiannual surveillance of the high-risk population by ultrasound and alpha-feto protein, HCC is increasingly detected at early stage, when curative treatments can be employed[3,4,7,8]. Resection is the mainstay of treatment for patients with HCC solitary tumours, preserved liver function, or mild portal hypertension not suitable for liver transplantation (LT)[9-11], the latter being the only cure of both HCC and underlying cirrhosis[12]. However, resection may be associated with significant morbidity as well as tumour recurrence, which occurs in about 70% of patients at 5 years[13-17]. When surgery is unfeasible, percutaneous or laparoscopic tumour ablation is the most widely used treatment that can achieve the complete local control of the disease in properly selected candidates[4,18,19]. This procedure is also cost-effective as compared to surgical treatments because it destroys only a minimal amount of liver parenchyma whilst reducing the number of hospitalizations[18,20,21]. Among the available local ablative techniques, laser ablation (LA) is a less investigated and little-used treatment. Our ambition is to provide hepatologists and other physicians with an updated approach to this ablative technique.
GENERAL PRINCIPLES
Laser source
In 1983, Bown[22] described for the first time the use of laser light to ablate liver tumours. Laser devices transform electrical energy into light energy, which interacts with tissue to produce heat and cause cell death[23]. Laser light can be delivered precisely and predictably into any location of the liver. Laser is an acronym for “light amplification by stimulated emission of radiation”, a principle based on the spontaneous emission of characteristic photons by excited atoms. Because laser light is coherent and monochromatic, it can be highly collimated and focused and large amounts of energy can be transmitted over long distances without significant losses. The light produced is of a specific wavelength and defines the properties of the laser system and the extent of tissue penetration. Due to the optimal penetration of light in the near-infrared spectrum, neodymium-doped yttrium aluminium garnet (Nd:YAG) lasers with a wavelength of 1064 nm and diode with a wavelength of 800-980 nm are preferred for percutaneous LA[24]. The optical (scattering, reflections, and absorption), thermal (conductivity and thermal storage), and blood flow characteristic of the tissue govern thermal diffusion processes and define the temperature map within the laser-exposed area[25]. The extent and completeness of tumour necrosis depends on a balance between the power applied and tissue charring[26].
Laser transmission
Laser light is transmitted from the source to the patient through flexible optic fibres that have specially designed diffuser tips. An important role is played by the shape, size, and design of the fibre[27-29]. The most common types of fibre currently used are the bare-tip[30,31] and cylindrical diffusing quartz fibres[29]. For the ablation of large masses or multiple tumours located at different sites, beam-splitting devices allowing the simultaneous delivery of light into multiple fibres can be used[24,32,33]. Multi-fibre systems have a synergistic effect by reduced heat dissipation between fibres[24,32]. The use of water-cooled laser application sheaths allows operating at higher powers and makes large lesions faster. Lesion diameter approaches 5-8 cm[33-35] with minimal charring and carbonization[26,29,36-41]. Because there is no destruction of the fibre, multiple applications are generally quite easy and longer lesions can be generated by simply pulling the fibre back in the applicator or advancing it forward.
Role of imaging guidance
The ablation procedure is performed under conscious sedation and local anaesthesia. Real-time ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI) are employed to guide either one or multiple thin needle-fibres[42,43] or a coaxial guide needle through tissue and into lesion[44,45]. Most patients are treated as day-cases in outpatient clinics. The number of treatment sessions varies according to the size and number of lesions. Follow-up evaluations are performed within 24 to 48 h or within 4 wk from procedure, and then at 3 to 6 mo intervals as is usual with other thermal techniques[42,44].
US is used for targeting and monitoring during the procedure, while CT is mainly used for post-treatment assessment. Heated tissue becomes hyperechoic because of water loss; this is most pronounced when there is tissue charring[46,47], particularly evident when using uncooled devices as in the laser technique with thin needle-fibres[31,42]. As it is well known, the main disadvantage of US guidance is that it is not suitable to accurately evaluate the temperature or the size of the ablative zone being created[47,48]. Otherwise, the contrast-enhanced US is useful to detect residual disease during procedure[49,50].
Real-time CT is unreliable for the detection of the early signs of laser-induced tissue injury. However, contrast enhanced CT 24 h after the procedure identifies coagulation zone as a not perfused area and correlates precisely with histology. The main role is the detection of residual or recurrent tumour following LA. Within a few days from treatment, the edges of ablation zone become indistinct due to inflammatory changes. During follow-up, local recurrences are easily visualized as contrast-enhancing foci adjacent to the necrotic area[30,51-53].
In contrast, MRI performed during LA allows the monitorization of the actual size and temperature of the ablation zone. MRI is the most accurate method for planning, monitoring, controlling, and assessing laser-induced coagulative necrosis[54-56]. LA power settings and session-treatment durations can be adjusted to obtain appropriate temperature elevations beyond tumour margins, thereby achieving a sufficient safety margin of necrosis. MRI is well suited to detecting residual undamaged tissue or local recurrence in the transition area[57]. This procedure is mainly used in combination with the high power water-cooled laser systems so that the treatment can be performed safely and with a better control of the extent of the ablated area[40,58]. MRI images can be acquired in near real time in any arbitrary plane. This has advantages in optimal planning of the procedure and in more accurately targeting the treatment volume and avoiding damages to critical structures[59]. After treatment delivery, changes in parameters such as tissue perfusion or diffusion may be used in addition to routine relaxation mechanisms (T1 and T2 weighting) to visualize the extent of ablation. Thus, because modern LA delivery aims to generate lesions rapidly in tissue that has many connective heat sinks and critical structures (such as brain and prostate), the ability to visualize and often quantify tissue temperature changes can be crucial feedback to the safety, efficacy, and overall outcomes of the thermal procedures[24,59,60].
Indications
According to the procedure used and the accessible facilities, selection criteria vary among centers[31,42,44,53,61-65] being established on size, number, and site of HCC in patients who are considered not good candidates for resection or liver transplantation. Although lesions of up to 6 cm have been treated[43], patients eligible for LA are those whose tumours are in accordance with the Milan criteria, irrespective of their location[66-68]. In fact, cancers located near major vessels, bile ducts, bowel, or diaphragm can be ablated with caution with RFA[69] but they can be more safely treated both with MRI-guided technique[44] and, more easily, with very thin devices with a calibre of 0.7 mm (21 gauge)[42,43,53,65-68]. MRI-guided technique allows confident ablation of high-risk located lesions using the real time thermometry and multiplanar MRI targeting[44,58,61,63].
Effectiveness and outcome data
Several retrospective cohort studies have shown that LA is a safe and feasible procedure for the treatment of HCC[31,42-44,53,61-68]. Using multiple bare fibres introduced through 21-gauge needles positioned under US-guidance, the reported complete response rate ranges from 82% to 97%[66-68]. In lesions in high-risk sites, complete response is 95.5%[65]. In patients with monofocal HCC ≤ 4 cm or three nodules ≤ 3 cm each, reported cumulative survival rates at 3 and at 5 years range from 52% to 68% and from 15% to 34%, respectively[53,66-68]. Tumor size, tumor location, and complete ablation were the main factors affecting the outcomes. In a multicenter study, Child’s class A patients had a 5-year cumulative survival of 41%; the median survival time was 65 and 68 mo in patients with tumor size ≤ 3 cm and ≤ 2 cm, respectively. The authors stated that the ideal candidates for LA are younger patients with serum albumin within the normal range and a tumor size ≤ 2 cm in whom it is very likely that complete ablation will be achieved. The median time to recurrence was 24 mo and the median disease-free survival time was 26 mo[68]. Like RFA and microwaves ablation (MWA), LA resulted safe and effective also in the treatment of cirrhotic patients awaiting liver transplantation[70].
Promising results have been reported with the use of water-cooled higher power MRI-guided LA. A very low local recurrence rate and a complete response rate reaching up to 98% in nodules ≤ 5 cm has been achieved with this technique[44,61]. In a study on 39 patients with 61 HCCs a complete ablation rate of 98% and a mean survival rate of 4.4 years were observed[61]. More recently, the same authors confirmed the high percentage of complete response in a cohort of 113 patients with 175 HCCs ≤ 5 cm followed for a period of over 15 years; 75% of the lesions were located at high-risk sites and median survival was 3.5 years[44].
To date there is only one controlled study comparing LA with RFA in treating a small cohort of patients (81 cirrhotic patients with 95 biopsy-proven HCCs) with early stage HCC (nodule ≤ 4.0 cm or three nodules ≤ 3.0 cm each). Thin multiple fibre technique to perform LA and single or cluster 17-gauge cool-tip electrodes for RFA were employed. The authors found LA and RFA to be equally effective; but fewer treatment sessions were needed in RFA group to achieve complete response. Neither significant differences in survival rates between the two methods nor significant complications were observed in both groups[71].
In a randomized prospective trial in a single centre with three years of follow-up being evaluated for final publication, the authors treated 140 patients with 157 biopsy-proven HCCs to compare LA and RFA (70 patients with 77 nodules and 70 patients with 80 nodules, respectively). Median follow up in RFA and LA groups was 21 and 22.5 mo, respectively. Complete response was observed in 97.2% and in 95.8% of RFA and LA group patients, respectively. Median time to tumour recurrence was 25.6 and 37.8 mo in RFA and in LA groups, respectively (P = 0.129). Estimated probability of survival at 1, 2, and 3 years was 94%, 88%, and 66% in RFA group and 94%, 81%, and 59% in LA group, respectively (P = 0.693). No major complications or significant treatment-related morbidity were observed in both groups. The authors concluded that LA was non inferior to RFA either in obtaining the complete ablation of HCC nodules or in long-term outcome[72].
Use in combination with other treatment
Multi-ablation therapy consisting of LA before trans-arterial-chemo-embolization (TACE) has been effective in large HCCs with a mean diameter of 5.2 cm (range, 3.1-9.6 cm)[73], with complete response achieved in 90% of the large tumors. Fifteen additional synchronous small HCC ≤ 3 cm in 11 patients were completely ablated (100%) with LA alone. The survival rate was overall 40% at 3 years and 60% in Child class A patients. The 1-, 2-, and 3-year local recurrence rate for the main tumors was 7% annually while the 1- and 2-year cancer-free survival rates were 74% and 34%, respectively. The rationale of this study was that LA reduces tumor volume within the range of TACE effectiveness and at same time can achieve complete destruction of large lesions with a lower number of TACE sessions (in 70% of patients only a single TACE session was done). Recently, the introduction in clinical practice of a novel needle guide system makes it possible to achieve complete ablation of nodules up to 5 cm in a single session in 91.7% of cases without resorting to combined treatment[43]. All data reported above are summarized in Table 1.
Table 1.
Studies reporting the outcome of Laser Ablation for small hepatocellular carcinoma
| Ref. | Pts/Tumors no | Tumor size (cm) mean | Complete ablationa, % | Local recurrence rate, % |
Overall survival % |
3-yr disease-free survival % | Major complication rateb, % | Mortality rate, % | P value | |
| 3-yr | 5-yr | |||||||||
| Giorgio et al[31] (2000) | 77/85 | ≤ 4.0c | 82f | 1.1 | 3.9d | 1.3d | ||||
| Pacella et al[73] (2001) | 30/30 | > 5.0 | 90f (+ TACE) | 7 | 40 | 0 | 0 | |||
| 30/15 | ≤ 3.0 | 100f | 0 | 0 | 0 | |||||
| Child-Pugh A | 60 | 0.001 | ||||||||
| Child-Pugh B | 0 | |||||||||
| Pacella et al[66] (2001) | 74/92 | ≤ 4.0c | 97f | 6 | 68 | 15 | 0 | 0 | ||
| Child-Pugh A | 73 | 31 | 0.052 | |||||||
| Child-Pugh B7,8,9 | 68 | 0 | ||||||||
| Eichler et al[61] (2001) | 39/61 | ≤ 5.0e | 97.5g | 0 | 4.4 yr | 0 | 0 | |||
| Pacella et al[42] (2005) | 82/99 | ≤ 4.0c | 90.9f | 8.8 | 1.5 | 0 | ||||
| Francica et al[64] (2007) | 148/169 | ≤ 4.0c | 82f | 14.7 | 52 | 27 | 0.6 | 0.6 | ||
| ≤ 2.0 | 95f | 5 | ||||||||
| ≤ 3.0 | 89f | 15 | ||||||||
| > 3.0 | 74f | 26 | 0.001 | |||||||
| Well-differentiated | ≤ 3.0 | 0 | 58 | |||||||
| Poorly-differentiated | 25 | 0.008 | ||||||||
| Pacella et al[68] (2009)1 | 432/548 | ≤ 4.0c | 79.6f | 202 | 61 | 34 | 1.6 | 0.2 | ||
| ≤ 2.0 | 85.1f | 41 | ||||||||
| Child-Pugh A | 63 | |||||||||
| Child-Pugh A | ≤ 2.0 | 683 | ||||||||
| Francica et al[53] (2012) | 106/1164 | ≤ 4.0c | 92.2f | 10.6 | 0.9 | 0.5 | NS | |||
| 58/66 | ≤ 4.0c | 95.5f | ||||||||
| Francica et al[65] (2012) | 116/132 | ≤ 4.0c | 100f | 18.0 | 57 | 29 | 0.0295 | |||
| 2.25 | ||||||||||
| Eichler et al[44] (2012) | 113/175 | ≤ 5.0e | 98g | 1.1 | 54 | 30 | 0 | 0 | ||
| Di Costanzo et al[43] (2013) | 104/116 | ≤ 6.0 | 87.6f | 16 | ||||||
| ≤ 5.0 | 91.7f | |||||||||
| Di Costanzo et al[72] (2013)6 | 70/80 (LA) | ≤ 5.06 | 96.3f | 23.9 | 66 | 42 | 0 | 0 | ||
| 70/77 | ≤ 5.06 | 97.4f | 25.7 | 59 | 43 | 0 | 0 | 0.693 | ||
Calculated per tumor;
Calculated per patient;
Single tumor ≤ 4 cm or ≤ 3 nodules each ≤ 3 cm;
In patients with Child-Pugh C;
Single tumor ≤ 5 cm or multiple ≤ 5;
With bare fibers;
With water-cooled fibers;
Multicentric retrospective study;
Local and distant;
In pts Child-Pugh class A well-differentiated tumor;
At risk site;
Only if the ablative margin was ≥ 7.5 mm;
RCT with Milan criteria;
Refers to the Child-Pugh class. NA: Not available; NS: Nonsignificant; CR: Complete response.
LA may also be combined with other modalities to achieve an increased volume of tumor necrosis. Zou et al[74] demonstrated that combined therapy with PEI immediately followed by LA resulted in a significantly larger volume of coagulation zone with reduced residual tumor volume on rabbit VX2 liver tumors. These authors hypothesized that tissue destruction by ethanol may have resulted in increased thermal conduction. In addition, the sclerosis and/or destruction induced on small vessels by PEI causes a reduction of the heat-sink effect and thus an enhancement of laser ablation effect. To date there are no clinical trials with this technique.
Complications
Arienti et al[75] performed a multicenter study involving nine centers in Italy with 520 patients who underwent 1064 nm laser sessions for 647 HCCs. Analyzing 90 factors for each record, including tumour characteristics, the authors reported a major complications rate of 1.5% (0.8% death rates) and a minor complications rate of 6.2%. These authors enrolled in their retrospective and prospective study patients with HCC nodules of any size [387 (60%) small, 180 (28%) intermediate, and 74 (11%) large] including 29 (5.9%) patients in Child’s class C and 72.1% of patients with portal hypertension. Major complications were associated with excess energy deposition and high-risk nodule locations. Minor complications proved to be associated with excess energy, high bilirubin level, and low prothrombin time. The authors who use MRI-guidance and high-calibre water-cooled devices reported no major complications or cases of mortality in 152 patients treated using large bore water-cooled devices[44,61]. In these series, no case of tumour seeding was observed.
Costs
Using multiple small-bore needles, the price of each laser disposable kit including a needle and a fiber is about €300 (US$ 400). Therefore, the cost of a single LA session varies in relation to the number of devices used: one kit is required for nodules ≤ 1.0 cm; 2 kits for nodules ranging from 1.0 to 2.0 cm, and 4 kits for larger nodules (Figure 1). Treatment can be performed in outpatient surgery by an operator, a nurse, and an anaesthesiologist and requires about 30-45 min (from targeting to final US assessment).
Figure 1.
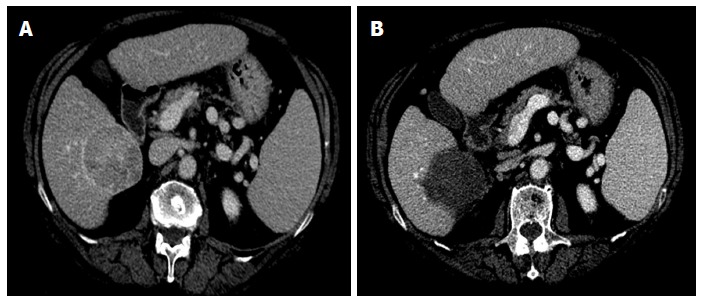
Representative case of complete ablation of large nodule with multi-fibre technique. A: Computed tomography (CT) scan before laser ablation (LA) session shows a nodular lesion 6 cm in maximum diameter (hepatocellular carcinoma moderately differentiated) localized in the S6 with exophytic growth (exophytic component > 40%); B: CT scan performed 4 wk after LA procedure shows complete necrosis of the tumor. Four illuminations were performed using the pullback technique and the treatment lasted 24 min. The procedure was well tolerated and the patient was discharged from the hospital 24 h after the procedure. The only side effects were mild pain and self-limiting fever lasting for 7 d.
TRANSLATING ALL THE AFOREMENTIONED INTO CLINICAL PRACTICE: TOWARDS A PATIENT-TAILORED APPROACH
Before commenting on the role that laser technology plays in percutaneous ablation of HCC, we should briefly summarize the recommended indications commonly accepted by the scientific community so far. On the basis of published data, RFA is now the first-line ablative technique whenever possible[76-81]. PEI has less local control effectiveness but still has a role in achieving complete response when the residual untreated viable tissue is minimal or when location at risk-sites implies serious adverse events or severe complications[82-85]. For solitary HCC ≤ 2 cm, RFA should be considered the first-line treatment for its lower mortality and morbidity, shorter hospitalization, and lower costs (compared to surgery), and should be preferred to PEI due to its greater effectiveness and predictability of treatment results[86]. Survival outcomes of patients with HCC < 3 cm treated by percutaneous approach are competitive with those of surgery. However, a careful multidisciplinary evaluation of the age and comorbidities of the patients and of the location of these tumours is needed[82,84]. In HCC > 3 cm resection or combined treatment (TACE + RFA or PEI) has been suggested to improve survival[87,88], but available studies do not yet provide useful conclusions as the enrollment criteria of patients was too stringent[88]. Studies are needed to define which population can benefit from the combined treatments.
As RFA effectiveness is size-dependent, to obtain complete necrosis the upper limits must not exceed 2.5-3.0 cm[89,90]. To overcome this limitation and obtain larger volumes of necrosis, a variety of devices[91,92] of different shapes and designs[93,94] used either with different algorithms[95] or activated in different modes (consecutive, simultaneous, or switching) has been developed[96-98]. In the treatment of large HCC (≥ 5 cm), conventional RFA is limited mainly by incomplete ablation, with reported complete ablation rate of 74% after single session in lesions between 3 and 5 cm and of 62% in tumours > 5 cm after multiple sessions[99]. Using three internally cooled bipolar electrodes complete, ablation rates was 81% in patients with large HCC[100].
Therefore, multiple heat sources are needed to obtain large volumes of necrosis; the laser technique with multiple thin needle fibres and simultaneous approach[42] satisfies this need. Indeed, LA obtains interesting results with thin, very simple devices that are much less sophisticated and less expensive than those used by RFA. According to the size and shape of the lesions, one to four fibers are used. Two laser fibers for nodules ≤ 2.0 cm and four fibers with tips arranged in a square configuration for larger nodules are used. For a single illumination, laser light is employed for 4-6 min. For nodules > 3.0 cm, multiple illuminations and the pullback technique are employed. The introduction of the novel needle guide has made it possible to obtain a complete ablation of lesions up to 5 cm[43]. No specific methods are used for treating lesions in high-risk (i.e., near gallbladder, main biliary duct, hepatic hilum, adjacent hollow viscera, or exophytic location) and/or hard-to-reach locations (e.g., in the dome of the liver, in the caudate lobe)[42,43,65]. Additionally, this technique makes it is relatively easy to obtain a safety margin ≥ 5 mm in a higher percentage of cases (62%)[53] than that reported by other authors with RFA[101-105]. Furthermore, thin devices makes it possible to treat multiple lesions of the liver of different sizes and in different locations in the same LA session without increasing the complications rate[43]. Therefore, it is possible to customize the ablative treatment according to the size and location of the lesion to be treated. Laser techniques can be used effectively in patients with very early and early HCC (BCLC 0 and A) because of their high percentage of complete response. The reported local effectiveness and long-term outcomes obtained with LA are comparable with those of RFA. Specifically, in the subgroup of Child’s class A cirrhotic patients with lesions ≤ 2 cm (BCLC 0-A) without contraindications to surgery treated by LA, 5-year survival was equivalent to that of RFA[68,78] as reported above. Finally, thanks to thin needles and to the more effective tumoricidal action of heat compared to ethanol, we believe that this technique could replace PEI in the treatment of nodules at high-risk sites when RFA is not technically feasible, as has been recommended by some authors[106].
As for the water-cooled laser applicators, it must be emphasized that their main advantage is their MRI compatibility, which allows pre-procedure planning and intraprocedure treatment monitoring using a variety of temperature-sensitive techniques[107,108]. The Frankfurt group has provided compelling long-term survival data in patients treated with this method for the ablation of hepatic metastases[109] and has recently published two papers on primary liver lesions in cirrhotic patients with a high percentage of complete response and low local recurrence[44] (Table 1). However, to achieve these excellent results, the authors used a large cross-sectional probe diameter (3 mm) that requires large bore cannula (9 gauge) for percutaneous treatment. In addition, the diffusion of MRI-guided LA is restricted by machine availability and by complexity of the procedure, requiring between 60 and 120 min to be completed[110,111]. New MRI-compatible applicators permit the execution of the whole procedure within the MR suite, reducing the procedural time and increasing technical effectiveness[112]. However, we think that although interventional MRI guidance is undoubtedly more accurate than US for monitoring ablation, its use would greatly limit the number of centers capable of performing tumor ablation, with ablation procedures being relegated to only those facilities with such specialized equipment. Thus, given that US is readily available, its use has proven to be successful on a practical level in these last 20 years, compared to the potential benefits of less available technologies. In short, these data show that touted advantages of a particular system do not have equal weight in the clinical scenario. Last but not least, we must add the costs of this option to its overall complexity.
A new ablation laser system consisting of 980-nm diode laser with a power of 15-W and diffuser-tipped optical fiber inserted through a 17-gauge internally cooled catheter was recently introduced in field practice. This system achieves a large, well-circumscribed ellipsoid ablation zone up to 2.0 cm × 2.3 cm in a single application lasting about three minutes, and up to 3.7 cm × 3.2 cm with two parallel applicators placed 1.5 cm apart[60]. Due to its characteristics, this system has been applied thus far to focal malignant lesions of the prostate and the brain[24,59]; research and clinical applications on hepatic focal lesions are underway (oral communication). Therefore, the limitations of the previous system, which used high-calibre devices, can be overcome by this technical solution. Further, the execution time of the entire manoeuvre can be shortened significantly by using real-time RM guidance.
Again, ex vivo and in vivo studies are underway (unpublished data) using diffuser-tipped optical fiber that can be placed in the target area through flexible internally cooled catheter under US guidance. It is possible to produce areas of necrosis of about 3.5 cm × 3.0 cm× 3.0 cm in diameter in about 20 min. If these data are confirmed by clinical studies, we will have made good use of the advantages of US guidance in combination with those deriving from a caliber similar to that of RFA- and MWA-cooled electrode. Therefore, with laser technical improvements such as the new small cylindrical diffuser[60] or the novel needle guide system[43], it possible to employ an array of applicators to increase the ablation zone without increasing invasiveness, procedural complexity, times of ablation, or costs. In clinical practice, a trade-off must be made between these multiple factors and the operator’s skill, the available technology, and the biology of the tumor.
While the reported outcome data with combined treatment (LA plus TACE) are interesting, they were obtained with a technique that is the opposite[73] of what is commonly used in referral centers. When surgery is unfeasible, a combined/sequential approach (PEI plus RFA, TACE plus PEI, RFA or MW) should be considered on an individual basis for multinodular nodules and for nodules > 3 cm, after multidisciplinary evaluation[85]. Recently, a meta-analysis of RFA following TACE reported no significant difference in survival rates between RFA plus TACE and RFA for small HCC. On the contrary, this sequential treatment improved overall survival rate in patients with intermediate and large HCC[113]. Therefore, the main indication of combined therapies is for lesions > 3 cm and < 8 cm. Both the PEI and TACE with different mechanism cause a reduction of the blood flow through the tumor, thereby facilitating a larger ablation zone.
LA before TACE, instead, reduces the tumor burden and brings the lesion back within the range of TACE effectiveness. In other words, LA results in a minimal amount of tumor tissue, which can be destroyed with selective TACE using a lesser amount of embolizing material (Figure 2). Because it is possible to destroy lesions up to 5-6 cm with laser technique (Figure 1) we think that this combined method might be effective in treating lesions larger than 6 cm both in cirrhotic patients and in non-cirrhotic patients, thereby avoiding surgery, as currently suggested by some authors[114].
Figure 2.
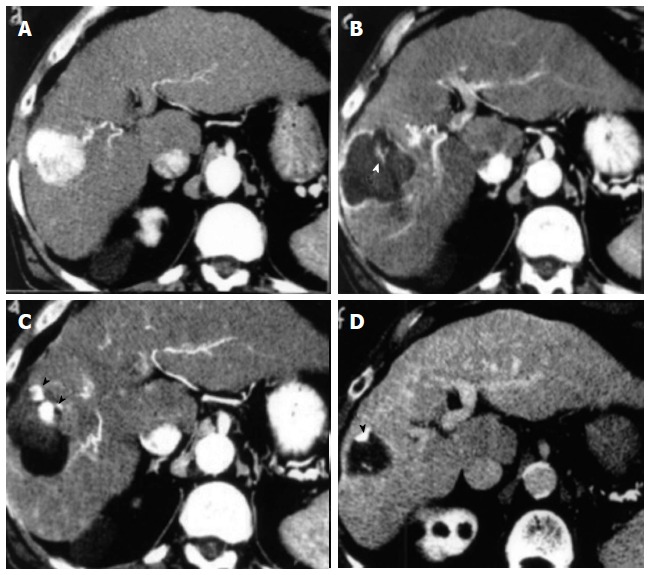
Representative case of complete ablation of hepatocellular carcinoma of 5 cm with combined treatment (laser ablation followed by trans-arterial-chemo-embolization). A: Computed tomography (CT) scan before Laser ablation (LA) shows a lesion 5 cm in diameter beneath the capsule in S8 during arterial phase; B: CT scan after LA shows an area of necrosis larger than basal lesion with small viable foci (white arrowhead) within the zone of coagulation; C: CT scan shows compact retention of iodized oil in the residual viable tissue (black arrowheads) after trans-arterial-chemo-embolization (TACE) session; D: CT scan shows marked volume reduction of treated area and clear shrinkage of viable tissue (black arrowhead) 6 mo after the combined procedure.
The safety of the procedure was investigated in a multicenter study sufficiently representative both of the type and of the number of possible complications when using either multiple thin needles[75] or large water-cooled devices[44]. The data reported above compare favourably with those of the tested and much more widely used RFA technique and with those of the MWA technique. The mortality rates of RFA range from 0% to 1.5% of cases and major complications from 1.5% to 5.8% of cases[69,115-118]. The mortality and major complications rates of MWA have been reported as 0% to 5.1% and 2.6% to 5.1%, respectively[119-122].
Finally, a few words regarding the MWA technique: its safety profile appears good, but there is still no confirmation on large series of cirrhotic patients. In the only comparative study with RFA, MWA showed comparable therapeutic efficacy and complications rates than RFA, but required more treatment sessions. Furthermore, adequate clinical data are lacking[123].
CONCLUSION
Given that there is not a single method available that meets all the requirements of an ideal ablation system, based on what has been discussed above and on data from the vast literature available, we can reasonably draw some conclusions: (1) the differences between the techniques in terms of the results are modest; (2) one technique may be more difficult than another and more rapid than another. In other words, there are differences in the ease and duration of the various procedures; and (3) while some energy sources may be better suited to certain applications, none has proven suitable for all applications. The laser technique developed in the United Kingdom has been used in the last two decades mainly in German and in Italy but has not been commercialized and sponsored in the rest of the world[124,125]. We hope that in the future a greater availability of the applicators will facilitate their use in clinical practice. The technique has been sufficiently tested and the recent RCT trial should validate it. The fine needle technique offers maximum flexibility, thereby allowing a tailored approach to the characteristics of each nodule in any location of each patient. More in general, we think that the reference centres that treat more than 50 patients/year should be equipped with all the available techniques so as to be able to use the best and the most suitable for each type of lesion for each patient.
Footnotes
P- Reviewer: Tiribelli C S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1. Available from: http://globocaniarcfr 2008.
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 7.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 8.Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol. 2003;39:1076–1084. doi: 10.1016/s0168-8278(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 10.Lang BH, Poon RT, Fan ST, Wong J. Perioperative and long-term outcome of major hepatic resection for small solitary hepatocellular carcinoma in patients with cirrhosis. Arch Surg. 2003;138:1207–1213. doi: 10.1001/archsurg.138.11.1207. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 13.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 14.Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10:S46–S52. doi: 10.1002/lt.20044. [DOI] [PubMed] [Google Scholar]
- 15.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 16.Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, Cescon M, Ravaioli M, Grazi GL, Bolondi L, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 17.Forner A, Bruix J. Ablation for hepatocellular carcinoma: Is there need to have a winning technique? J Hepatol. 2010;52:310–312. doi: 10.1016/j.jhep.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 19.Bolondi L, Cillo U, Colombo M, Craxì A, Farinati F, Giannini EG, Golfieri R, Levrero M, Pinna AD, Piscaglia F, et al. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45:712–723. doi: 10.1016/j.dld.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, Rodés J, Bruix J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Bown SG. Phototherapy in tumors. World J Surg. 1983;7:700–709. doi: 10.1007/BF01655209. [DOI] [PubMed] [Google Scholar]
- 23.Jacques SL. Laser-tissue interactions. Photochemical, photothermal, and photomechanical. Surg Clin North Am. 1992;72:531–558. doi: 10.1016/s0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 24.Stafford RJ, Fuentes D, Elliott AA, Weinberg JS, Ahrar K. Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng. 2010;38:79–100. doi: 10.1615/critrevbiomedeng.v38.i1.70. [DOI] [PubMed] [Google Scholar]
- 25.Svaasand LO, Boerslid T, Oeveraasen M. Thermal and optical properties of living tissue: application to laser-induced hyperthermia. Lasers Surg Med. 1985;5:589–602. doi: 10.1002/lsm.1900050607. [DOI] [PubMed] [Google Scholar]
- 26.Sturesson C. Interstitial laser-induced thermotherapy: influence of carbonization on lesion size. Lasers Surg Med. 1998;22:51–57. doi: 10.1002/(sici)1096-9101(1998)22:1<51::aid-lsm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Wyman DR, Whelan WM, Wilson BC. Interstitial laser photocoagulation: Nd: YAG 1064 nm optical fiber source compared to point heat source. Lasers Surg Med. 1992;12:659–664. doi: 10.1002/lsm.1900120615. [DOI] [PubMed] [Google Scholar]
- 28.Moller PH, Lindberg L, Henriksson PH, Persson BR, Tramberg KG. Interstitial laser thermotherapy: comparison between bare fibre and sapphire probe. Laser Med Sci. 1996;10:193–200. [Google Scholar]
- 29.Heisterkamp J, van Hillegersberg R, Sinofsky E, JN IJ. Heat-resistant cylindrical diffuser for interstitial laser coagulation: comparison with the bare-tip fiber in a porcine liver model. Lasers Surg Med. 1997;20:304–309. doi: 10.1002/(sici)1096-9101(1997)20:3<304::aid-lsm9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 30.Amin Z, Donald JJ, Masters A, Kant R, Steger AC, Bown SG, Lees WR. Hepatic metastases: interstitial laser photocoagulation with real-time US monitoring and dynamic CT evaluation of treatment. Radiology. 1993;187:339–347. doi: 10.1148/radiology.187.2.8475270. [DOI] [PubMed] [Google Scholar]
- 31.Giorgio A, Tarantino L, de Stefano G N, Catalano O, Cusati B, Del Viscovo L A, Caturelli E. Interstitial laser photocoagulation under ultrasound guidance of liver tumors: results in 104 treated patients. Eur J Ultrasound. 2000;11:181–188. doi: 10.1016/s0929-8266(00)00086-0. [DOI] [PubMed] [Google Scholar]
- 32.Steger AC, Lees WR, Shorvon P, Walmsley K, Bown SG. Multiple-fibre low-power interstitial laser hyperthermia: studies in the normal liver. Br J Surg. 1992;79:139–145. doi: 10.1002/bjs.1800790215. [DOI] [PubMed] [Google Scholar]
- 33.Heisterkamp J, van Hillegersberg R, Sinofsky E, Ijzermans JN. Interstitial laser phocoagulation with four cylindrical diffusing fbre tips: importance of mutual fibre distance. Lasers Med Sci. 1999;14:216–220. [Google Scholar]
- 34.Nolsøe CP, Torp-Pedersen S, Burcharth F, Horn T, Pedersen S, Christensen NE, Olldag ES, Andersen PH, Karstrup S, Lorentzen T. Interstitial hyperthermia of colorectal liver metastases with a US-guided Nd-YAG laser with a diffuser tip: a pilot clinical study. Radiology. 1993;187:333–337. doi: 10.1148/radiology.187.2.8475269. [DOI] [PubMed] [Google Scholar]
- 35.Vogi T, Mack MG, Straub R, Roggan A, Felix R. Percutaneous MRI-guided laser-induced thermotherapy for hepatic metastases for colorectal cancer. Lancet. 1997;350:29. doi: 10.1016/s0140-6736(97)24027-4. [DOI] [PubMed] [Google Scholar]
- 36.Godlewski G, Rouy S, Pignodel C, Ould-Said H, Eledjam JJ, Bourgeois JM, Sambuc P. Deep localized neodymium (Nd)-YAG laser photocoagulation in liver using a new water cooled and echoguided handpiece. Lasers Surg Med. 1988;8:501–509. doi: 10.1002/lsm.1900080509. [DOI] [PubMed] [Google Scholar]
- 37.Van Hillegersberg R, Kort WJ, ten Kate FJ, Terpstra OT. Water-jet-cooled Nd: YAG laser coagulation: selective destruction of rat liver metastases. Lasers Surg Med. 1991;11:445–454. doi: 10.1002/lsm.1900110510. [DOI] [PubMed] [Google Scholar]
- 38.van Hillegersberg R, de Witte MT, Kort WJ, Terpstra OT. Water-jet-cooled Nd: YAG laser coagulation of experimental liver metastases: correlation between ultrasonography and histology. Lasers Surg Med. 1993;13:332–343. doi: 10.1002/lsm.1900130310. [DOI] [PubMed] [Google Scholar]
- 39.Vogl TJ, Mack MG, Müller PK, Straub R, Engelmann K, Eichler K. Interventional MR: interstitial therapy. Eur Radiol. 1999;9:1479–1487. doi: 10.1007/s003300050874. [DOI] [PubMed] [Google Scholar]
- 40.Vogl TJ, Eichler K, Straub R, Engelmann K, Zangos S, Woitaschek D, Böttger M, Mack MG. Laser-induced thermotherapy of malignant liver tumors: general principals, equipment(s), procedure(s)--side effects, complications and results. Eur J Ultrasound. 2001;13:117–127. doi: 10.1016/s0929-8266(01)00125-2. [DOI] [PubMed] [Google Scholar]
- 41.Veenendaal LM, de Jager A, Stapper G, Borel Rinkes IH, van Hillegersberg R. Multiple fiber laser-induced thermotherapy for ablation of large intrahepatic tumors. Photomed Laser Surg. 2006;24:3–9. doi: 10.1089/pho.2006.24.3. [DOI] [PubMed] [Google Scholar]
- 42.Pacella CM, Bizzarri G, Francica G, Bianchini A, De Nuntis S, Pacella S, Crescenzi A, Taccogna S, Forlini G, Rossi Z, et al. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with small tumors: analysis of factors affecting the achievement of tumor necrosis. J Vasc Interv Radiol. 2005;16:1447–1457. doi: 10.1097/01.rvi.90000172121.82299.38. [DOI] [PubMed] [Google Scholar]
- 43.Di Costanzo GG, D’Adamo G, Tortora R, Zanfardino F, Mattera S, Francica G, Pacella CM. A novel needle guide system to perform percutaneous laser ablation of liver tumors using the multifiber technique. Acta Radiol. 2013;54:876–881. doi: 10.1177/0284185113489825. [DOI] [PubMed] [Google Scholar]
- 44.Eichler K, Zangos S, Gruber-Rouh T, Vogl TJ, Mack MG. Magnetic resonance-guided laser-induced thermotherapy in patients with oligonodular hepatocellular carcinoma: long-term results over a 15-year period. J Clin Gastroenterol. 2012;46:796–801. doi: 10.1097/MCG.0b013e3182641806. [DOI] [PubMed] [Google Scholar]
- 45.Vogl TJ, Huebner F, Naguib NN, Bauer RW, Mack MG, Nour-Eldin NE, Meister D. MR-based thermometry of laser induced thermotherapy: temperature accuracy and temporal resolution in vitro at 0.2 and 1.5 T magnetic field strengths. Lasers Surg Med. 2012;44:257–265. doi: 10.1002/lsm.22012. [DOI] [PubMed] [Google Scholar]
- 46.Gertner MR, Wilson BC, Sherar MD. Ultrasound properties of liver tissue during heating. Ultrasound Med Biol. 1997;23:1395–1403. doi: 10.1016/s0301-5629(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 47.Malone DE, Wyman DR, DeNardi FG, McGrath FP, De Gara CJ, Wilson BC. Hepatic interstitial laser photocoagulation. An investigation of the relationship between acute thermal lesions and their sonographic images. Invest Radiol. 1994;29:915–921. doi: 10.1097/00004424-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Tranberg KG, Möller PH, Hannesson P, Stenram U. Interstitial laser treatment of malignant tumours: initial experience. Eur J Surg Oncol. 1996;22:47–54. doi: 10.1016/s0748-7983(96)91451-1. [DOI] [PubMed] [Google Scholar]
- 49.Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51 Suppl:S19–S23. doi: 10.1016/j.ejrad.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 50.Solbiati L, Tonolini M, Cova L. Monitoring RF ablation. Eur Radiol. 2004;14 Suppl 8:P34–P42. doi: 10.1007/s10406-004-0089-y. [DOI] [PubMed] [Google Scholar]
- 51.Amin Z, Thurrell W, Spencer GM, Harries SA, Grant WE, Bown SG, Lees WR. Computed tomography-pathologic assessment of laser-induced necrosis in rat liver. Invest Radiol. 1993;28:1148–1154. doi: 10.1097/00004424-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Harries SA, Amin Z, Smith ME, Lees WR, Cooke J, Cook MG, Scurr JH, Kissin MW, Bown SG. Interstitial laser photocoagulation as a treatment for breast cancer. Br J Surg. 1994;81:1617–1619. doi: 10.1002/bjs.1800811118. [DOI] [PubMed] [Google Scholar]
- 53.Francica G, Petrolati A, Di Stasio E, Pacella S, Stasi R, Pacella CM. Influence of ablative margin on local tumor progression and survival in patients with HCC ≤4 cm after laser ablation. Acta Radiol. 2012;53:394–400. doi: 10.1258/ar.2012.110471. [DOI] [PubMed] [Google Scholar]
- 54.de Jode MG, Vale JA, Gedroyc WM. MR-guided laser thermoablation of inoperable renal tumors in an open-configuration interventional MR scanner: preliminary clinical experience in three cases. J Magn Reson Imaging. 1999;10(4):545–549. doi: 10.1002/(sici)1522-2586(199910)10:4<545::aid-jmri7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 55.Morrison PR, Jolesz FA, Charous D, Mulkern RV, Hushek SG, Margolis R, Fried MP. MRI of laser-induced interstitial thermal injury in an in vivo animal liver model with histologic correlation. J Magn Reson Imaging. 1998;8:57–63. doi: 10.1002/jmri.1880080114. [DOI] [PubMed] [Google Scholar]
- 56.Joarder R, de Jode M, Lamb GA, Gedroyc WM. The value of MnDPDP enhancement during MR guided laser interstitial thermoablation of liver tumors. J Magn Reson Imaging. 2001;13:37–41. doi: 10.1002/1522-2586(200101)13:1<37::aid-jmri1006>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 57.Isbert C, Ritz JP, Schilling A, Roggan A, Heiniche A, Wolf KJ, Müller G, Buhr HJ, Germer CT. Laser induced thermotherapy (LITT) of experimental liver metastasis-detection of residual tumors using Gd-DTPA enhanced MRI. Lasers Surg Med. 2002;30:280–289. doi: 10.1002/lsm.10041. [DOI] [PubMed] [Google Scholar]
- 58.Dick EA, Wragg P, Joarder R, de Jode M, Lamb G, Gould S, Gedroyc WM. Feasibility of abdomino-pelvic T1-weighted real-time thermal mapping of laser ablation. J Magn Reson Imaging. 2003;17:197–205. doi: 10.1002/jmri.10242. [DOI] [PubMed] [Google Scholar]
- 59.Stafford RJ, Shetty A, Elliott AM, Klumpp SA, McNichols RJ, Gowda A, Hazle JD, Ward JF. Magnetic resonance guided, focal laser induced interstitial thermal therapy in a canine prostate model. J Urol. 2010;184:1514–1520. doi: 10.1016/j.juro.2010.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahrar K, Gowda A, Javadi S, Borne A, Fox M, McNichols R, Ahrar JU, Stephens C, Stafford RJ. Preclinical assessment of a 980-nm diode laser ablation system in a large animal tumor model. J Vasc Interv Radiol. 2010;21:555–561. doi: 10.1016/j.jvir.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eichler K, Mack MG, Straub R, Engelmann K, Zangos S, Woitaschek D, Vogl TJ. Oligonodular hepatocellular carcinoma (HCC): MR-controlled laser-induced thermotherapy. Radiologe. 2001;41:915–922. doi: 10.1007/s001170170063. [DOI] [PubMed] [Google Scholar]
- 62.Christophi C, Muralidharan V. Treatment of hepatocellular carcinoma by percutaneous laser hyperthermia. J Gastroenterol Hepatol. 2001;16:548–552. doi: 10.1046/j.1440-1746.2001.02477.x. [DOI] [PubMed] [Google Scholar]
- 63.Dick EA, Joarder R, de Jode M, Taylor-Robinson SD, Thomas HC, Foster GR, Gedroyc WM. MR-guided laser thermal ablation of primary and secondary liver tumours. Clin Radiol. 2003;58:112–120. doi: 10.1053/crad.2002.1129. [DOI] [PubMed] [Google Scholar]
- 64.Francica G, Iodice G, Delle Cave M, Sarrantonio R, Lapiccirella G, Molese V, Smeraldo D, Scarano F, De Marino F. Factors predicting complete necrosis rate after ultrasound-guided percutaneous laser thermoablation of small hepatocellular carcinoma tumors in cirrhotic patients: a multivariate analysis. Acta Radiol. 2007;48:514–519. doi: 10.1080/02841850701199942. [DOI] [PubMed] [Google Scholar]
- 65.Francica G, Petrolati A, Di Stasio E, Pacella S, Stasi R, Pacella CM. Effectiveness, safety and local progression after percutaneous laser ablation foe HCC nodules ≤ 4 cm are not affected by tumor location. AJR Am J Roentgenol. 2012;199:1393–1401. doi: 10.2214/AJR.11.7850. [DOI] [PubMed] [Google Scholar]
- 66.Pacella CM, Bizzarri G, Magnolfi F, Cecconi P, Caspani B, Anelli V, Bianchini A, Valle D, Pacella S, Manenti G, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712–720. doi: 10.1148/radiol.2213001501. [DOI] [PubMed] [Google Scholar]
- 67.Pacella CM, Bizzarri G, Francica G, Forlini G, Petrolati A, Valle D, Anelli V, Bianchini A, Nuntis SD, Pacella S, et al. Analysis of factors predicting survival in patients with hepatocellular carcinoma treated with percutaneous laser ablation. J Hepatol. 2006;44:902–909. doi: 10.1016/j.jhep.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 68.Pacella CM, Francica G, Di Lascio FM, Arienti V, Antico E, Caspani B, Magnolfi F, Megna AS, Pretolani S, Regine R, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27:2615–2621. doi: 10.1200/JCO.2008.19.0082. [DOI] [PubMed] [Google Scholar]
- 69.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]
- 70.Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19:7515–7530. doi: 10.3748/wjg.v19.i43.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrari FS, Megliola A, Scorzelli A, Stella A, Vigni F, Drudi FM, Venezia D. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med. 2007;112:377–393. doi: 10.1007/s11547-007-0148-2. [DOI] [PubMed] [Google Scholar]
- 72.Di Costanzo G, Tortora R, D’Adamo G, Addario L, Galeotta Lanza A, Lampasi F, De Luca M, Zanfardino F, Tartaglione MT, Mattera S, et al. Radiofrequency Ablation versus Laser Abaltion for the Treatment of Small Hepatocelluar Carcinoma in Cirrhosis: A Randomized Controlled Trial (Washington, ILCA Conference 2013; 0-028) Washington: ILCA Conference; 2013. pp. 18–19 (Abstract book). [Google Scholar]
- 73.Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669–678. doi: 10.1148/radiology.219.3.r01ma02669. [DOI] [PubMed] [Google Scholar]
- 74.Zou X, Liu Q, Zhou X, He G, Yu M, Han Z, Meng X, Su H. Ultrasound-guided percutaneous laser and ethanol ablation of rabbit VX2 liver tumors. Acta Radiol. 2013;54:181–187. doi: 10.1258/ar.2012.110723. [DOI] [PubMed] [Google Scholar]
- 75.Arienti V, Pretolani S, Pacella CM, Magnolfi F, Caspani B, Francica G, Megna AS, Regine R, Sponza M, Antico E, et al. Complications of laser ablation for hepatocellular carcinoma: a multicenter study. Radiology. 2008;246:947–955. doi: 10.1148/radiol.2463070390. [DOI] [PubMed] [Google Scholar]
- 76.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lü MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, Li DM, Lai JM, Li SQ. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yixue Zazhi. 2006;86:801–805. [PubMed] [Google Scholar]
- 78.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 79.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 80.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 82.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 83.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514–524. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 84.Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380–388. doi: 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Meza-Junco J, Montano-Loza AJ, Liu DM, Sawyer MB, Bain VG, Ma M, Owen R. Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev. 2012;38:54–62. doi: 10.1016/j.ctrv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Xie X, Dendukuri N, McGregor M. Percutaneous radiofrequency ablation for the treatment of early stage hepatocellular carcinoma: a health technology assessment. Int J Technol Assess Health Care. 2010;26:390–397. doi: 10.1017/S0266462310001029. [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741–749. doi: 10.1111/j.1478-3231.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- 88.Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 89.Ikeda K, Seki T, Umehara H, Inokuchi R, Tamai T, Sakaida N, Uemura Y, Kamiyama Y, Okazaki K. Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol. 2007;31:485–491. [PubMed] [Google Scholar]
- 90.Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, Tso WK, Fan ST, Poon RT. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20–29. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 91.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency using multiprobe arrays. Acad Radiol. 1995;2:670–674. [PubMed] [Google Scholar]
- 92.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 93.Mulier S, Ni Y, Miao Y, Rosière A, Khoury A, Marchal G, Michel L. Size and geometry of hepatic radiofrequency lesions. Eur J Surg Oncol. 2003;29:867–878. doi: 10.1016/j.ejso.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 94.Furse A, Miller BJ, McCann C, Kachura JR, Jewett MA, Sherar MD. Radiofrequency coil for the creation of large ablations: ex vivo and in vivo testing. J Vasc Interv Radiol. 2012;23:1522–1528. doi: 10.1016/j.jvir.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 95.Hope WW, Arru JM, McKee JQ, Vrochides D, Aswad B, Simon CJ, Dupuy DE, Iannitti DA. Evaluation of mulitprobe radiofrequency technology in a porcine model. HPB (Oxford) 2007;9:363–367. doi: 10.1080/13651820701611218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laeseke PF, Sampson LA, Haemmerich D, Brace CL, Fine JP, Frey TM, Winter TC, Lee FT. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241:116–124. doi: 10.1148/radiol.2411051271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, Choi BI. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 98.Brace CL, Sampson LA, Hinshaw JL, Sandhu N, Lee FT. Radiofrequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol. 2009;20:118–124. doi: 10.1016/j.jvir.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guglielmi A, Ruzzenente A, Battocchia A, Tonon A, Fracastoro G, Cordiano C. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480–484. [PubMed] [Google Scholar]
- 100.Seror O, N’Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, Coderc E, Trinchet JC, Beaugrand M, Sellier N. Large (& gt; or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology. 2008;248:288–296. doi: 10.1148/radiol.2481071101. [DOI] [PubMed] [Google Scholar]
- 101.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, Tsuchihashi T, Saigenji K. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 103.Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol. 2008;190:1544–1551. doi: 10.2214/AJR.07.2798. [DOI] [PubMed] [Google Scholar]
- 104.Peng ZW, Zhang YJ, Chen MS, Liang HH, Li JQ, Zhang YQ, Lau WY. Risk factors of survival after percutaneous radiofrequency ablation of hepatocellular carcinoma. Surg Oncol. 2008;17:23–31. doi: 10.1016/j.suronc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (& gt; 2 and & lt; 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 106.Lencioni R, Llovet JM. Percutaneous ethanol injection for hepatocellular carcinoma: alive or dead? J Hepatol. 2005;43:377–380. doi: 10.1016/j.jhep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Kickhefel A, Rosenberg C, Weiss CR, Rempp H, Roland J, Schick F, Hosten N. Clinical evaluation of MR temperature monitoring of laser-induced thermotherapy in human liver using the proton-resonance-frequency method and predictive models of cell death. J Magn Reson Imaging. 2011;33:704–712. doi: 10.1002/jmri.22499. [DOI] [PubMed] [Google Scholar]
- 108.Stollberger R, Ascher PW, Huber D, Renhart W, Radner H, Ebner F. Temperature monitoring of interstitial thermal tissue coagulation using MR phase images. J Magn Reson Imaging. 1998;8:188–196. doi: 10.1002/jmri.1880080132. [DOI] [PubMed] [Google Scholar]
- 109.Vogl TJ, Straub R, Eichler K, Söllner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;230:450–458. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 110.Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions) Radiology. 2002;225:367–377. doi: 10.1148/radiol.2252011171. [DOI] [PubMed] [Google Scholar]
- 111.Puls R, Langner S, Rosenberg C, Hegenscheid K, Kuehn JP, Noeckler K, Hosten N. Laser ablation of liver metastases from colorectal cancer with MR thermometry: 5-year survival. J Vasc Interv Radiol. 2009;20:225–234. doi: 10.1016/j.jvir.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 112.Puls R, Stroszczynski C, Rosenberg C, Kuehn JP, Hegenscheid K, Speck U, Stier A, Hosten N. Three-dimensional gradient-echo imaging for percutaneous MR-guided laser therapy of liver metastasis. J Magn Reson Imaging. 2007;25:1174–1178. doi: 10.1002/jmri.20936. [DOI] [PubMed] [Google Scholar]
- 113.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 116.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 117.Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–2596. doi: 10.1007/s00330-011-2222-3. [DOI] [PubMed] [Google Scholar]
- 118.Poggi G, Riccardi A, Quaretti P, Teragni C, Delmonte A, Amatu A, Saini G, Mazzucco M, Bernardo A, Palumbo R, et al. Complications of percutaneous radiofrequency thermal ablation of primary and secondary lesions of the liver. Anticancer Res. 2007;27:2911–2916. [PubMed] [Google Scholar]
- 119.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933–940. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 120.Ong SL, Gravante G, Metcalfe MS, Strickland AD, Dennison AR, Lloyd DM. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605. doi: 10.1097/MEG.0b013e328318ed04. [DOI] [PubMed] [Google Scholar]
- 121.Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 122.Poggi G, Montagna B, DI Cesare P, Riva G, Bernardo G, Mazzucco M, Riccardi A. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res. 2013;33:1221–1227. [PubMed] [Google Scholar]
- 123.Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 124.Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walser EM. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with a tumor size of 4 cm or smaller: analysis of factors affecting the achievement of tumor necrosis. J Vasc Interv Radiol. 2005;16:1427–1429. doi: 10.1097/01.RVI.0000188755.61481.E8. [DOI] [PubMed] [Google Scholar]


