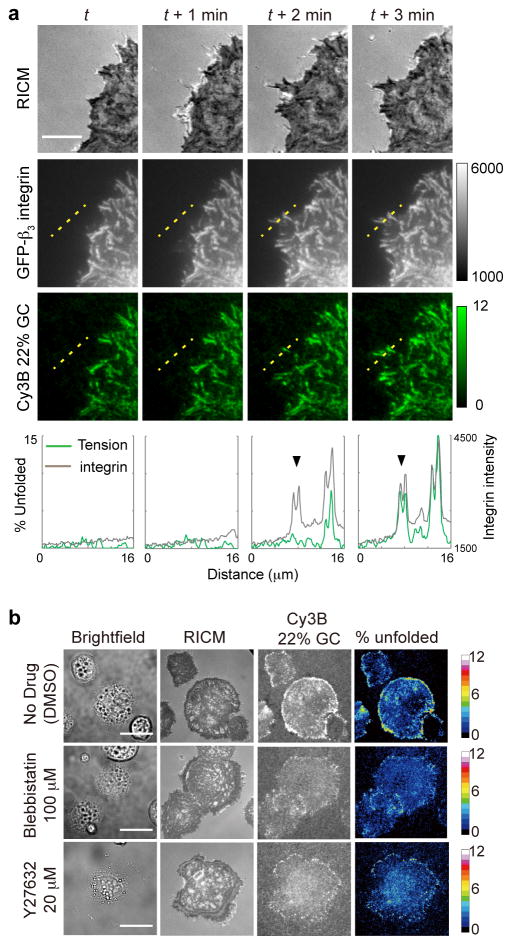
Fig. 3. Control experiments show that tension sensor response is mechanically mediated by integrin receptors.
(a) Colocalization of the integrin tension signal with GFP-β3-integrin fluorescence. Rat embryonic fibroblast (REF) cells transfected with GFP-β3-integrin were plated on a surface that encoded the 22% GC content probe with Cy3B. The images show RICM, GFP and Cy3B channels acquired over four timepoints. The intensity of the Cy3B channel corresponds to the fraction of 22% GC content hairpins that were unfolded under the cell. Linescan analysis of the two fluorescence channels is shown below each timepoint and show the arrival of the integrin receptors followed by the increase of integrin tension. This indicates that the majority of observed tension signal is transduced through the β3-integrin receptor. Scale bar: 10 μm. (b) Blocking myosin II activity modulates integrin mechanical tension during initial cell spreading and adhesion. Representative images in brightfield, RICM, and Cy3B channels for control cells as well as cells treated with myosin II inhibitors blebbistatin and the ROCK inhibitor Y27632. The fourth column of images represents the fraction of 22% GC hairpins unfolded under tension and is quantified using a color-coded heat map. Images were acquired 20 min after cell seeding on cRGDfK-modified tension probes. Scale bar: 10 μm