Abstract
AIM: To investigate the feasibility and safety of pH capsule to monitor pH in patients with gastroesophageal reflux disease (GERD).
METHODS: Ninety-one patients with symptoms suggestive of GERD were enrolled in this study, 46 of whom were randomized to the pH capsule group; the remaining 45 patients used the conventional catheter and pH capsule simultaneously. The pH data and traces were recorded via automatic analysis, and capsule detachment was assessed using X-ray images. All of the patients were required to complete a questionnaire regarding tolerance with the capsule.
RESULTS: The capsules were successfully attached on the first attempt, and no early detachment of the capsules was observed. Compared to the 24-h pH data recorded with the conventional catheter, the data collected with the pH capsule showed no significant differences in 24-h esophageal acid exposure. The measurements of esophageal acid exposure over 24 h collected with the two devices showed a significant correlation (r2 = 0.996, P < 0.001). Capsule detachment occurred spontaneously in 89 patients, and 2 capsules required endoscopic removal due to chest pain. The capsule was associated with less interference with daily activity.
CONCLUSION: The wireless pH capsule provides a feasible and safe method for monitoring gastroesophageal reflux and therefore may serve as an important tool for diagnosing GERD.
Keywords: Wireless pH capsule, Feasibility, Safety, Conventional catheter, Gastroesophageal reflux disease
Core tip: The new pH capsule (JSPH-1, made by Jinshan Science and Technology Co. Ltd, Chongqing, China), a wireless pH capsule developed in China, was introduced as an alternative method for pH monitoring. Compared with the result recorded synchronously with MMS catheter, that obtained with JSPH-1 pH capsule was of uniformity. Our study suggested that JSPH-1 pH capsule is feasible, safe, well tolerated for monitoring reflux in patients with gastroesophageal reflux disease (GERD), therefore it may serve as an important tool for the diagnosis of GERD.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or adverse events[1]. This is a common condition with a prevalence rate of 8.1%-27.8% in North America, 8.8%-25.9% in Europe, 2.5%-7.8% in East Asia, 8.7%-33.1% in the Middle East, 11.6% in Australia, and 23.0% in South America[2]. Moreover, GERD profoundly affects societies in terms of economic cost[3], quality of life, and working ability[4]. According to previous investigations, the mean total cost per patient over a 12-mo follow-up period was €5237, of which €4674 (89%) was due to lost work productivity in France[5]; GERD resulted in a 6% to 40% reduction in workers’ efficiency[6]. Therefore, the burden of GERD has promoted various efforts to identify appropriate diagnostic approaches and improve diagnostic modalities. Recent studies also suggest that the prevalence of GERD in Asia is increasing[7].
Esophageal pH monitoring is a valuable tool for the diagnosis and management of GERD. Conventional ambulatory esophageal 24-h pH monitoring is considered as the gold standard for diagnosing GERD and provides an objective diagnosis[8]. However, this measuring device is passed transnasally, can lead to discomfort and often requires patients to modify their daily activities or diets. Moreover, changing positions can lead to the migration of the catheter electrode, and these changes may potentially result in false-negative results[9-11]. In addition, esophageal acid exposure shows day-to-day variability and fluctuation, contributing to the inadequacy of this 24-h monitoring catheter system to record the extent of reflux events and related symptoms[12].
A new wireless technique, which was introduced by Medtronic, represents a significant advancement in better tolerability and higher sensitivity in the diagnosis of GERD[13-15]. Recently, with the improved understanding of GERD among patients and an increased demand for advanced diagnostic techniques in China, a new wireless pH capsule device (JSPH-1), was developed (Jinshan Science and Technology Co. Ltd, Chongqing, China).
In an effort to promote the clinical application of the JSPH-1 pH capsule, the present study was designed to assess the clinical feasibility and safety of the JSPH-1 pH capsule in patients with symptoms suggestive of GERD.
MATERIALS AND METHODS
Patients
Between December 2010 and June 2011, we enrolled patients with symptoms suggestive of GERD from the inpatient and outpatient departments of 4 university hospitals in China. Before enrollment, each patient had undergone routine hematology and coagulation tests, and an electrocardiographic examination. Patients were instructed to stop taking proton pump inhibitors for 7 d, histamine 2 receptor antagonists for 5 d, and antacids for at least 24 h prior to the start of the study procedures. The inclusion and exclusion criteria are listed in Table 1. The enrollment and allocation flow of patients with GERD is shown in Figure 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Age ≥ 18 yr and ≤ 65 yr |
| Typical symptoms: regurgitation and heartburn |
| Atypical symptoms: belching, chest pain, pharyngeal burning, foreign body sensations, chronic unexplained pharyngitis, hoarseness, bronchitis, and asthma |
| Proton pump inhibitor therapy-positive |
| Written informed consent |
| Exclusion criteria |
| Nasopharynx or upper esophagus obstruction |
| Esophageal varices and severe esophageal mucosal erosion |
| Severe esophageal motility disorder (e.g., achalaisa, scleroderma, diabetes mellitus, autonomic or peripheral neuropathy, and myopathy) |
| In vivo congenital gastrointestinal malformation, gastrointestinal obstruction, perforation, stricture, or fistula |
| A recent history of stomach surgery or gastrointestinal bleeding (within the past 6 mo) |
| A history of bleeding tendency and taking anticoagulant drugs in the long term |
| Any implanted electrical device, such as cardiac pacemakers |
| Allergy to the polymer materials |
| Various acute enteritis, severe ischemic disease, or radioactive colitis |
| Pregnancy/lactation |
| Unstable cardiopathy, psychotic diseases arrhythmia cordis, or being uncooperative |
Figure 1.
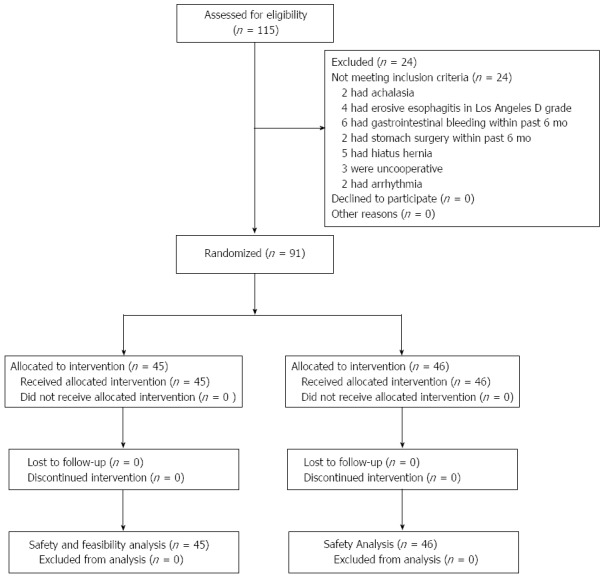
Enrollment and allocation of patients with gastroesophageal reflux disease.
All patients provided written informed consent to participate in the study. The protocol was approved by the ethics committees at each participating center (approval number: 2010-013) and the respective national authorities, as applicable. All authors had access to the study data and approved the statement. The trial was also registered in an independent clinical trial database (www.chictr.org; ID: ChiCTR-DDT-12002374).
Study design
This was a prospective, randomized, self-controlled, parallel-group, multicenter trial. The randomization sequence was generated by computer. The coded treatment assignments were kept at the coordinating center in sealed, consecutively numbered, opaque envelopes. Randomized assignments to the study groups were made by contacting the coordinating center by telephone.
Monitoring system
Esophageal pH data and traces were simultaneously monitored for 24 h using each system. The conventional catheter pH measurement system (MMS-Medical Measurement Systems BV, The Netherlands) used a transnasal antimony catheter.
The JSPH-1 pH capsule (Jinshan Science and Technology Co. Ltd, Chongqing, China) consisted of a capsule attached to the end of a catheter delivery system, a portable receiver, and a computer workstation (Figure 2A). The pH capsule was attached to the esophageal mucosa through a vacuum connection hole fixed to a suction pump, which was released from the delivery system by triggering the activation knobs in sequence (Figure 2B). The capsule was rectangular in shape (26.5 mm × 5.5 mm × 6 mm) and weighed 1.4 g. The pH sensors (dry antimony electrode and reference electrode) were on the distal tip, and an internal battery and transmitter were settled within the capsule (Figure 2C). The life span of the battery exceeded 96 h, and the pH data were transmitted to a receiver via radiofrequency telemetry every 15 s and were recorded at 3-s sampling intervals (frequency 0.33 Hz).
Figure 2.
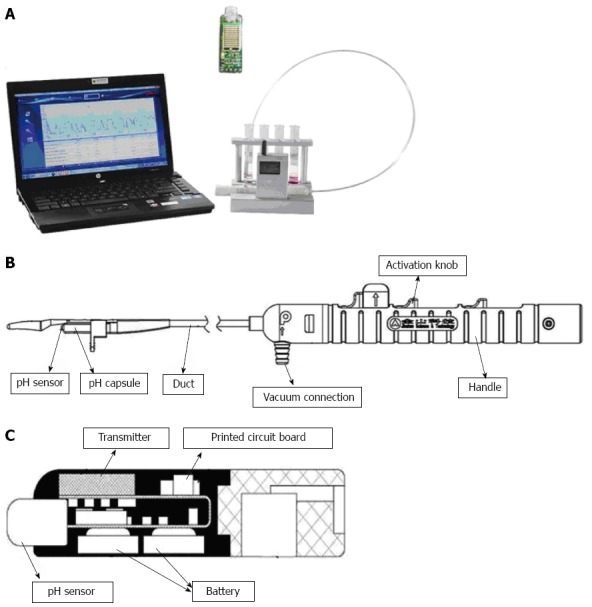
JSPH-1 pH capsule measurement system. A: The system includes a JSPH-1 delivery system, a receiver, a pH capsule, and a computer workstation; B: Prepackaged pH capsule incorporating both a delivery system and a pH capsule. The handle of the delivery system is separated from the capsule by triggering the activation knobs; C: The pH sensors (antimony pH electrode and reference electrode) are located on the distal tip of the capsule, and an internal battery, a transmitter, and a printed circuit board are contained within the capsule.
Esophageal pH monitoring
Prior to pH monitoring, the pH capsules were energized by removing the magnetic switches and then calibrated in buffer solutions (Sandhill Scientific, Inc., Highlands Ranch, CO, United States) of pH 1.07, pH 4.00, and pH 7.01. Endoscopy was performed under topical sedation with lidocaine hydrochloride mucilage. During the procedure, the distance between the squamocolumnar junction (SCJ) and the incisors was measured. The delivery system was smeared with lubricant and then transorally delivered to the calibrated location in the esophagus. To attach the capsules to the mucosa, the vacuum gauge of the pump was stabilized at 0.08 MPa for approximately 10 s. After the activation knobs on the handle were triggered in sequence, the pH capsule was released from the delivery system. Under vacuum pressure suction, the pH capsule could attach to the esophageal mucosa with the help of the clamping device.
The catheter pH electrode was calibrated in buffer solutions of pH 1.07 and pH 7.01. After pH capsule placement, the catheter electrode was passed transnasally and placed at the same level as the pH capsule. As the catheter pH electrode was fixed, an X-ray was obtained to observe the location of the 2 electrodes in order to confirm that they were at the same level. Simultaneous pH recording was also initiated.
Recording protocol
During the first 24 h of recording, the patients were instructed to perform activities as normally as usual and to keep a diary to document meal times, food intake, and the time and type of any symptoms. At the end of the first 24 h, the catheter electrode was removed, and the capsule was retained for additional pH recording. The patients returned to the monitoring centers, where X-rays were collected to confirm capsule detachment.
At the end of the study, the pH data were downloaded from the receivers and analyzed using a standard computer software program (pH Capsule Data Analysis Workstation, Jinshan Science and Technology Co. Ltd, Chongqing, China).
In addition, the patients were instructed to complete questionnaires describing tolerability, related symptoms, and modifications of daily activities during pH capsule monitoring.
Study parameters
The summary data included a pH trace and statistics on the total number of reflux episodes, the number of episodes longer than 5 min, the longest reflux episode during which the pH was < 4.0, and the total time and percentage of the total time when the pH was < 4.0. Abnormal esophageal acid exposure was defined as the percentage of the total time with a pH < 4.0[16], with a diagnostic threshold of 4.2% for catheter-based studies and 4.4% for wireless studies[17,18].
All adverse events were documented. Symptoms related to capsule attachment were assessed by a diary, including foreign body sensations, chest pain, nausea, and vomiting. In addition, foreign body sensations, saliva swallowing discomfort, chest discomfort, chest pain, dysphagia for solids or liquids, nausea, and vomiting were assessed throughout the pH monitoring period. Procedural adverse events, such as mucosal trauma, severe bleeding, and esophageal perforation, were also evaluated.
Tolerance evaluation was assessed by the patients, who also recorded their level of satisfaction with the procedure. The patients also rated the interference with normal daily activities, diet, and sleeping as none, mild, moderate, or severe.
Estimation of sample size
In a previous study[19], esophageal pH monitoring with a wireless system was associated with significantly greater esophageal discomfort (43%) compared to traditional catheter system. In another study[20], 48% of patients using a wireless pH capsule complained of throat discomfort. Our unpublished pilot data showed that 2 of 22 patients (9%) complained of moderate chest pain or esophageal discomfort when using the JSPH-1 device. Our sample size calculations were based on an allowed error of 0.06 measured with the JSPH-1 pH capsule. Therefore, these data indicated that 88 patients would be adequate for the safety study. According to our unpublished preliminary results, it was projected that the standard deviation of the difference between the 2 techniques would be 0.77 and that the allowed error measured by the 2 techniques would be 0.39. Using a type I error of 5.0% (2-sided) with a power of 90%, 43 patients were planned for the feasibility study.
Statistical analysis
Statistical analyses were performed using SPSS software (version 13.0). All data are expressed as mean ± SE, percentages, medians, and ranges. Comparisons of the esophageal acid exposure measured during the 24 h of simultaneous recordings in the 45 patients with the 2 systems were analyzed using Wilcoxon signed rank tests. Diagnostic concordance was calculated by dividing the number of patients with the same diagnostic result from both methods by the total number of patients and the kappa value. The correlation of the pH data between the 2 devices was determined using simple regression analysis. In all analyses, P < 0.05 was considered statistically significant.
RESULTS
Study population
We approached 115 patients with symptoms suggestive of GERD for study eligibility. A total of 24 patients were excluded (Figure 1), and 91 patients entered this study. Forty-six of the 91 patients randomized to JSPH-1 group did not receive the conventional catheter pH measurement system (MMS) monitoring, and the remaining 45 patients used the conventional catheter pH measurement system (MMS) and the JSPH-1 simultaneously.
A total of 91 patients with a mean age of 45.2 ± 12.8 years (range: 21-65 years) were enrolled in the study. Of these, 49 (53.8%) were female (mean age: 47.0 ± 11.8 years), and 42 (46.2%) were male (mean age: 43.1 ± 13.8 years). The patients presented with heartburn (n = 14), regurgitation (n = 6), chest pain (n = 24), pharyngeal burning or foreign body sensations (n = 10), hoarseness (n = 1), heartburn and regurgitation (n = 23), and regurgitation and chest pain (n = 13).
Attachment
Placement of the pH capsule was successful in all the patients at the first attempt. As shown in Figure 3A, endoscopy indicated that the pH capsule electrode was attached 6 cm proximally to the SCJ. According to the prior study[21], recommended endoscopic pH capsule placement was 6 cm proximal to the SCJ, approximate to the conventional pH catheter placement positioned 5 cm proximal to the upper margin of the low esophageal sphincter (LES). Control X-rays revealed that the capsule and MMS catheter electrode were located at the same level in the esophagus (Figure 3B). After capsule detachment on day 1, endoscopy revealed only mild localized mucosal trauma at the site of attachment in all patients (Figure 3C). All patients successfully completed at least 24 h of wireless pH monitoring. The diagnostic efficacy was 100%.
Figure 3.
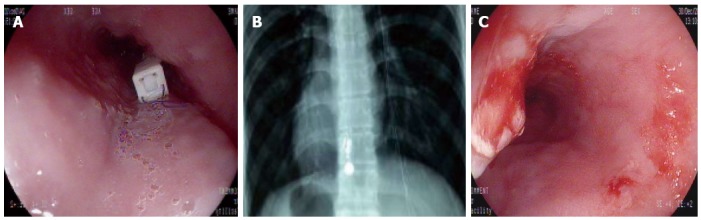
Attached pH capsule and the fixed location. A: Endoscopy showing the pH capsule attached 5 cm proximal to the squamocolumnar junction; B: X-ray showing the pH capsule electrode and the catheter pH electrode at the same level; C: Esophageal mucosa showing procedural mild trauma after capsule detachment.
Prolonged monitoring time: The 48-h recording rate was 88% (80/91). Capsule detachment occurred between 25 and 27 h in 10 patients, and incomplete data capture occurred in 1 patient. In addition, 38 of the 91 patients underwent 96-h wireless pH monitoring during the study period. Capsule detachment occurred between 48 and 72 h in 2 patients and between 72 and 96 h in 3 other patients. Thus, 96-h recordings were available in 33 (36%) patients. The overall mean recording time was 72 ± 24 h.
Tolerability
The pH capsules were generally well tolerated, and only 1 patient experienced strong discomfort due to capsule attachment, as observed by endoscopy. Eight-five patients (93.4%) stated that they would be willing to undergo the procedure with capsule again. In addition, 34 (37.4%) patients reported mild or moderate interference with normal daily activities, diet, and sleeping during the capsule monitoring period.
Safety
Forty-five (49.5%) patients experienced symptoms related to capsule attachment, including nausea in 29 (31.9%), foreign body sensations in 7 (7.7%), and chest pain in 11 (12.1%). Two (2.2%) patients experienced more than 1 symptom. Sixty patients (65.9%) experienced symptoms during the pH monitoring period, including discomfort when swallowing saliva in 13 (14.3%), dysphagia for solids in 21 (23.1%), foreign body sensations in 33 (36.3%), chest discomfort in 6 (6.6%), and chest pain in 3 (3.3%). Sixteen patients (17.6%) experienced more than 1 symptom.
No patients were lost to follow-up during the study period. According to X-rays, capsule detachment occurred spontaneously in 89 patients. The other 2 patients complained of intolerable chest pain during the pH monitoring period on day 7, which required endoscopic removal. No serious adverse events occurred. The capsule disappeared in all of the patients after capsule detachment on day 2.
Comparison of esophageal acid exposure monitored with JSPH-1 pH capsule and MMS-catheter
During the first 24-h period, esophageal acid exposure was monitored simultaneously with the JSPH-1 pH capsule and MMS catheter in 45 patients. Correct positioning of both probes was confirmed according to X-ray images in all patients.
The pH parameters of esophageal acid exposure recorded with both devices during the initial 24 h period are shown in Table 2. There were no significant differences in the 24-h esophageal acid exposure recorded with the 2 devices in terms of the total number of reflux episodes (P = 0.305), the number of episodes longer than 5 min (P = 0.058), the longest reflux time (P = 0.125), or the percentage of total time with a pH < 4.0 (P = 0.171) (Figure 4A-D). However, the median total duration with a pH < 4.0 recorded by the 2 devices was significantly different (P = 0.014; Figure 4E).
Table 2.
Comparison of esophageal acid exposure measured during 24 h of simultaneous recordings in 45 patients with pH capsule (JSPH-1) and the conventional pH measurement system
| Parameter | JSPH-1 | MMS | P-value1 |
| Reflux episodes (n) | 17 (8-36.5) | 16 (6-39.5) | 0.305 |
| Reflux episodes > 5 min (n) | 0 (0-2) | 1 (0-3.5) | 0.058 |
| Longest reflux time (min) | 5.4 (2.1-14.2) | 6.5 (1.8-15.4) | 0.125 |
| pH < 4, total time (min) | 14.6 (4.0-53.8) | 20 (5.6-54.1) | 0.014 |
| pH < 4, percentage of total time (%) | 1.2 (0.3-4.1)2 | 1.5 (0.5-4.0)2 | 0.171 |
Wilcoxon signed rank test;
Results are median (25th-75th). MMS: Measurement system.
Figure 4.
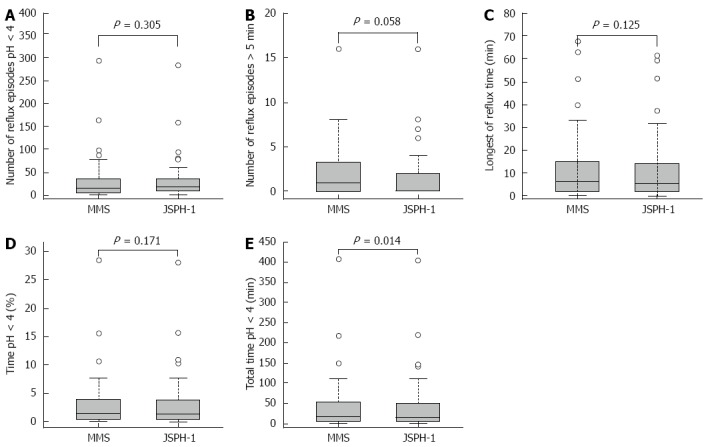
Comparison of esophageal acid exposure measured during 24 h of simultaneous recordings in 45 patients with the conventional pH measurement system and the pH capsule (JSPH-1). A-D: There were no significant differences in pH parameters including the number of reflux episodes with a pH < 4 (P = 0.305), the number of episodes longer than 5 min (P = 0.058), the longest reflux time (P = 0.125), or the percentage of total time with a pH < 4 (P = 0.171) between the 2 systems; E: Overall median value of the total time with a pH < 4 was significantly shorter with the capsule system than with the catheter system (P = 0.014).
The difference in the percentage of total time with a pH < 4.0 between the devices was less than 0.5%, and there was a strong significant correlation [r2 = 0.996, P < 0.001)] between the 24-h esophageal acid exposure recorded with the pH capsule and the MMS catheter (Figure 5A-E).
Figure 5.
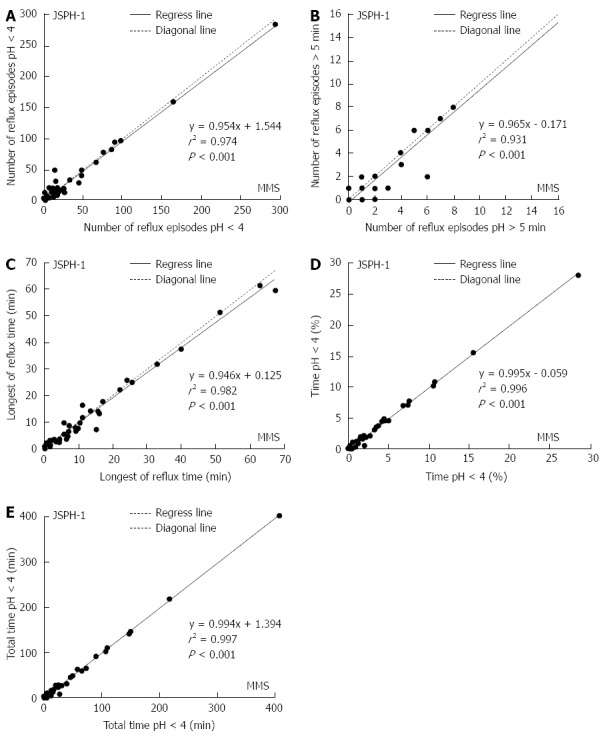
The 24-h esophageal acid exposure recorded simultaneously using the conventional pH measurement system and the pH capsule (JSPH-1). The figure shows the regression equation and the regression line (A-E). The indicated esophageal acid exposure over 24 h measured by the 2 devices showed a significant correlation.
Abnormal esophageal acid exposure was identified in the same 11 patients by the two methods. Therefore, 11 patients were diagnosed with reflux disease with two devices and the diagnostic concordance for GERD was 100% (κ = 1.000).
A comparison of the pH representative traces recorded with the 2 devices showed that the pattern and duration of acid reflux events were consistent (Figure 6).
Figure 6.
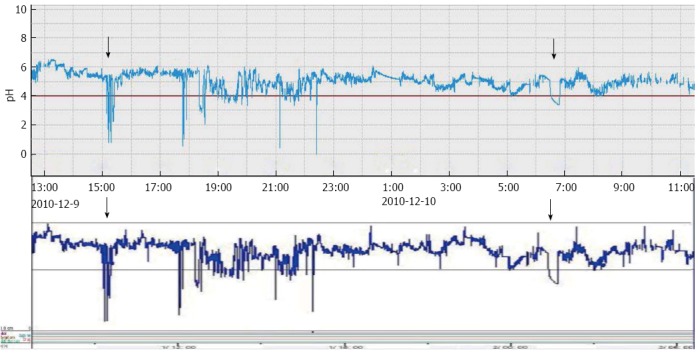
Representative traces of simultaneous recordings of esophageal pH with the pH capsule (JSPH-1) (upper trace) and the conventional pH measurement system (lower trace). Arrows indicate pH episodes detected simultaneously by the 2 devices. Both the pattern of contrast pH traces and the acid reflux period between the JSPH-1 and measurement system were consistent.
DISCUSSION
Wireless esophageal pH monitoring is a new diagnostic method for GERD and has shown some advantages over traditional methods. The Bravo pH monitoring system has proven to be a feasible, safe, and well-tolerated device for esophageal pH monitoring. Moreover, there is a strong correlation between the esophageal acid exposure recorded with the Bravo capsule and that with conventional catheter monitoring systems[22,23]. The JSPH-1 pH capsule system is a new wireless diagnostic tool developed by Jinshan Science and Technology Co. Ltd, Chongqing, China, which is slightly smaller and lighter than the Bravo capsule. The recording time of the JSPH-1 capsule is over 96 h, compared to Bravo capsule (24-48 h). Importantly, the sampling frequency of JSPH-1 capsule (1/3 s) was faster than that of Bravo capsule (1/6 s).
Simultaneous data recorded using the JSPH -1 pH capsule and the MMS catheter showed no significant differences in 24-h esophageal acid exposure, with a minimal difference of 0.5%. The placement of the pH capsule was successful, and evaluable 24-h recordings were available for all patients. We observed a statistically significant correlation between the acid exposure times recorded by the 2 devices, and the pH traces showed high levels of consistency. These findings support the feasibility of the JSPH-1 capsule for monitoring esophageal pH in patients with GERD. Moreover, our results with this device are similar to those reported with the Bravo wireless system[22,24].
It has previously been reported that wireless pH capsule monitoring improves tolerance and produces less interference with daily activities and nasopharyngeal discomfort than conventional catheter techniques[25-27]. The pH capsule is disposable and avoids the risk of cross-infection, which also increases its viability and popularity[28,29]. Our data further suggested that most patients achieved overall satisfaction with the system and would be willing to accept a pH capsule test again. The capsule was also associated with less impairment in daily activities and diet modifications. Importantly, no serious adverse events and no signs of gastrointestinal bleeding in terms of hematemesis or melena occurred during the capsule detachment period. Therefore, our results indicate that the JSPH-1 pH capsule was safe and well-tolerated.
As our study was conducted in 4 centers, it may in part reflect the real-life conditions of using the pH capsule. However, the administration of an objective questionnaire for monitoring adverse events during the JSPH-1 pH capsule monitoring period may have provided more relevant information. The main adverse events include throat discomfort, foreign body sensations, odynophagia, mucosal injury, chest pain, esophageal perforation, and aspiration[30-33]. In our study, the incidence of throat discomfort (14.3%) and foreign body sensations (36.3%) were similar to those in other reports[18,30], which varied between 4% and 14% and between 33% and 34%, respectively. In our study, 6 patients (6.6%) complained of chest discomfort, possibly due to the capsule causing mild injury at the site of suction. It has previously been reported that chest pain due to endoscopic removal of the capsule occurs in less than 4% of cases[34,35]. In our study, 2 (2.2%) patients with intolerable chest pain required endoscopic removal of the capsule, and this pain may have been caused by esophageal hypercontractility triggered as a result of capsule placement[36].
Our study confirms the feasibility and safety of the JSPH-1 pH capsule for esophageal pH monitoring, especially for patients with typical symptoms and negative endoscopic findings. Our study also demonstrates that the device is well tolerated for pH monitoring in patients with GERD. However, further study is required to identify an effective means of controlling capsule detachment in a predicted time, and it would also be beneficial to extend the battery life beyond 96 h, thereby enabling the device to be used for monitoring the effectiveness of treatment interventions for GERD.
COMMENTS
Background
Esophageal pH monitoring is a valuable tool for the diagnosis of gastroesophageal reflux disease (GERD). As conventional catheter pH monitoring produces discomfort and interferes with daily activities, a new wireless pH capsule was recently introduced as an alternative method for pH monitoring. This study assessed the feasibility and safety of pH capsule to monitor pH in patients with GERD.
Research frontiers
For its high prevalence and negative effect on quality of life, GERD is always the focus of the research. Esophageal pH monitoring is valuable for the diagnosis and management of GERD. Conventional ambulatory esophageal 24-h pH monitoring may lead to discomfort and often requires patients to modify their daily activities or diets. Moreover, changing positions can lead to the migration of the catheter electrode, and potentially results in false-negative results. In addition, the 24-h monitoring catheter system can not record day-to-day variability and fluctuation of esophageal acid exposure. So, it is necessary to develop a wireless esophageal pH capsule.
Innovations and breakthroughs
The pH capsules were successfully attached at the first attempt, and no early detachment of the capsules was observed. Compared to the conventional catheter, the data from pH capsule showed a significant correlation in 24-h esophageal acid exposure. The capsule was associated with less interference with daily activities.
Applications
The JSPH-1 pH capsule can be used as an alternative method for pH monitoring. Their results suggested JSPH-1 pH capsule is feasible, safe and well tolerated for monitoring reflux in patients with GERD, therefore it may serve as an important tool for the diagnosis of GERD.
Peer review
The authors assess the feasibility and safety of a new wireless pH capsule to monitor esophageal pH in GERD patients through carrying out a nice direct comparison between capsule and traditional pH studies. They concluded that the wireless pH capsule is a safe, effective and well-tolerated method for monitoring esophageal pH in patients with GERD, and showed a marginal advantage of the new device over others. This is a very interesting paper.
Footnotes
Supported by Grants from the Innovation Fund for Technology-Based Firms of the Ministry of Science and Technology of the China, No. 12C26215115999 (in part); Special Fund on Industrial Revitalization and Transformation of the National Development and Reform Commission of the China; and Jinshan Science and Technology Co. Ltd, Chongqing, China
P- Reviewer: Bordas JM, Chai J, Hashimoto N, Herszenyi L, McMahon BP S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. [The Montreal definition and classification of gastroesophageal reflux disease: a global, evidence-based consensus paper] Z Gastroenterol. 2007;45:1125–1140. doi: 10.1055/s-2007-963633. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87.e1-3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med. 1998;104:252–258. doi: 10.1016/s0002-9343(97)00354-9. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, Sandler RS. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 6.Wahlqvist P, Reilly MC, Barkun A. Systematic review: the impact of gastro-oesophageal reflux disease on work productivity. Aliment Pharmacol Ther. 2006;24:259–272. doi: 10.1111/j.1365-2036.2006.02996.x. [DOI] [PubMed] [Google Scholar]
- 7.Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14–27. doi: 10.5056/jnm.2011.17.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin MS. Esophageal pH and Combined Impedance-pH Monitoring in Children. Pediatr Gastroenterol Hepatol Nutr. 2014;17:13–22. doi: 10.5223/pghn.2014.17.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fass R, Hell R, Sampliner RE, Pulliam G, Graver E, Hartz V, Johnson C, Jaffe P. Effect of ambulatory 24-hour esophageal pH monitoring on reflux-provoking activities. Dig Dis Sci. 1999;44:2263–2269. doi: 10.1023/a:1026608804938. [DOI] [PubMed] [Google Scholar]
- 10.Aksglaede K, Funch-Jensen P, Thommesen P. Intra-esophageal pH probe movement during eating and talking. A videoradiographic study. Acta Radiol. 2003;44:131–135. doi: 10.1080/j.1600-0455.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 11.Schneider JH, Kramer KM, Königsrainer A, Granderath FA. Ambulatory pH: monitoring with a wireless system. Surg Endosc. 2007;21:2076–2080. doi: 10.1007/s00464-007-9301-1. [DOI] [PubMed] [Google Scholar]
- 12.Ahlawat SK, Novak DJ, Williams DC, Maher KA, Barton F, Benjamin SB. Day-to-day variability in acid reflux patterns using the BRAVO pH monitoring system. J Clin Gastroenterol. 2006;40:20–24. doi: 10.1097/01.mcg.0000190753.25750.0e. [DOI] [PubMed] [Google Scholar]
- 13.Gillies RS, Stratford JM, Booth MI, Dehn TC. Oesophageal pH monitoring using the Bravo catheter-free radio capsule. Eur J Gastroenterol Hepatol. 2007;19:57–63. doi: 10.1097/MEG.0b013e3280116eec. [DOI] [PubMed] [Google Scholar]
- 14.Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2005;3:329–334. doi: 10.1016/s1542-3565(05)00021-2. [DOI] [PubMed] [Google Scholar]
- 15.Ang D, Teo EK, Ang TL, Ong J, Poh CH, Tan J, Fock KM. To Bravo or not? A comparison of wireless esophageal pH monitoring and conventional pH catheter to evaluate non-erosive gastroesophageal reflux disease in a multiracial Asian cohort. J Dig Dis. 2010;11:19–27. doi: 10.1111/j.1751-2980.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325–332. [PubMed] [Google Scholar]
- 17.Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci. 1992;37:849–856. doi: 10.1007/BF01300382. [DOI] [PubMed] [Google Scholar]
- 18.Wenner J, Johnsson F, Johansson J, Oberg S. Wireless oesophageal pH monitoring: feasibility, safety and normal values in healthy subjects. Scand J Gastroenterol. 2005;40:768–774. doi: 10.1080/00365520510023602. [DOI] [PubMed] [Google Scholar]
- 19.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong WM, Bautista J, Dekel R, Malagon IB, Tuchinsky I, Green C, Dickman R, Esquivel R, Fass R. Feasibility and tolerability of transnasal/per-oral placement of the wireless pH capsule vs. traditional 24-h oesophageal pH monitoring--a randomized trial. Aliment Pharmacol Ther. 2005;21:155–163. doi: 10.1111/j.1365-2036.2005.02313.x. [DOI] [PubMed] [Google Scholar]
- 21.Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut. 1999;44:476–482. doi: 10.1136/gut.44.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Håkanson BS, Berggren P, Granqvist S, Ljungqvist O, Thorell A. Comparison of wireless 48-h (Bravo) versus traditional ambulatory 24-h esophageal pH monitoring. Scand J Gastroenterol. 2009;44:276–283. doi: 10.1080/00365520802588109. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfino JE, Schreiner MA, Lee TJ, Zhang Q, Boniquit C, Kahrilas PJ. Comparison of the Bravo wireless and Digitrapper catheter-based pH monitoring systems for measuring esophageal acid exposure. Am J Gastroenterol. 2005;100:1466–1476. doi: 10.1111/j.1572-0241.2005.41719.x. [DOI] [PubMed] [Google Scholar]
- 24.des Varannes SB, Mion F, Ducrotté P, Zerbib F, Denis P, Ponchon T, Thibault R, Galmiche JP. Simultaneous recordings of oesophageal acid exposure with conventional pH monitoring and a wireless system (Bravo) Gut. 2005;54:1682–1686. doi: 10.1136/gut.2005.066274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grigolon A, Bravi I, Cantù P, Conte D, Penagini R. Wireless pH monitoring: better tolerability and lower impact on daily habits. Dig Liver Dis. 2007;39:720–724. doi: 10.1016/j.dld.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Bradley AG, Crowell MD, DiBaise JK, Kim HJ, Burdick GE, Fleischer DE, Sharma VK. Comparison of the impact of wireless versus catheter-based pH-metry on daily activities and study-related symptoms. J Clin Gastroenterol. 2011;45:100–106. doi: 10.1097/MCG.0b013e3181e5d32a. [DOI] [PubMed] [Google Scholar]
- 27.Wenner J, Johnsson F, Johansson J, Oberg S. Wireless esophageal pH monitoring is better tolerated than the catheter-based technique: results from a randomized cross-over trial. Am J Gastroenterol. 2007;102:239–245. doi: 10.1111/j.1572-0241.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 28.Ward EM, Devault KR, Bouras EP, Stark ME, Wolfsen HC, Davis DM, Nedrow SI, Achem SR. Successful oesophageal pH monitoring with a catheter-free system. Aliment Pharmacol Ther. 2004;19:449–454. doi: 10.1111/j.1365-2036.2004.01868.x. [DOI] [PubMed] [Google Scholar]
- 29.Tankurt E, Tüzömay N, Sengül B, Kinik E, Açil B. Safety and tolerability of BRAVO capsule pH monitoring. Turk J Gastroenterol. 2011;22:352–353. doi: 10.4318/tjg.2011.0229. [DOI] [PubMed] [Google Scholar]
- 30.Fajardo NR, Wise JL, Locke GR, Murray JA, Talley NJ. Esophageal perforation after placement of wireless Bravo pH probe. Gastrointest Endosc. 2006;63:184–185. doi: 10.1016/j.gie.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Bhat YM, McGrath KM, Bielefeldt K. Wireless esophageal pH monitoring: new technique means new questions. J Clin Gastroenterol. 2006;40:116–121. doi: 10.1097/01.mcg.0000196188.57543.75. [DOI] [PubMed] [Google Scholar]
- 32.Pandolfino JE, Kahrilas PJ. Prolonged pH monitoring: Bravo capsule. Gastrointest Endosc Clin N Am. 2005;15:307–318. doi: 10.1016/j.giec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 33.von Renteln D, Kayser T, Riecken B, Caca K. An unusual case of Bravo capsule aspiration. Endoscopy. 2008;40 Suppl 2:E174. doi: 10.1055/s-2007-995804. [DOI] [PubMed] [Google Scholar]
- 34.Prakash C, Jonnalagadda S, Azar R, Clouse RE. Endoscopic removal of the wireless pH monitoring capsule in patients with severe discomfort. Gastrointest Endosc. 2006;64:828–832. doi: 10.1016/j.gie.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Pandolfino JE. Bravo capsule pH monitoring. Am J Gastroenterol. 2005;100:8–10. doi: 10.1111/j.1572-0241.2005.41183.x. [DOI] [PubMed] [Google Scholar]
- 36.Tharavej C, Hagen JA, Portale G, Hsieh CC, Gandamihardja TA, Lipham JC, Peters JH, DeMeester SR, Crookes PF, Bremner CG, et al. Bravo capsule induction of esophageal hypercontractility and chest pain. Surg Endosc. 2006;20:783–786. doi: 10.1007/s00464-005-0257-8. [DOI] [PubMed] [Google Scholar]


