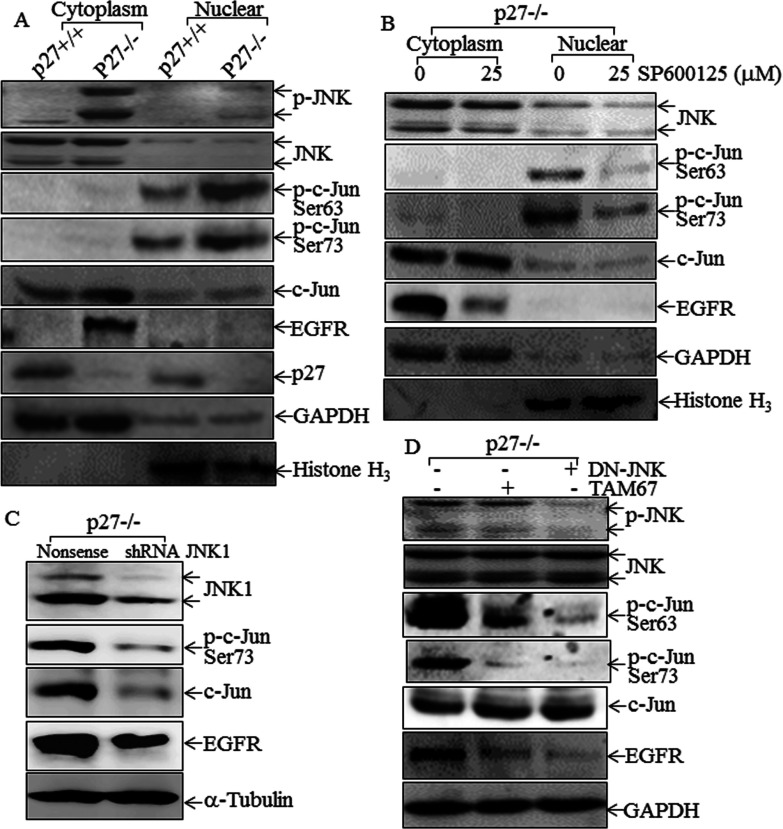
Figure 3. p27Kip1 inhibited EGFR expression by targeting JNK/c-Jun activation.
(A) Cytosolic and nuclear proteins were isolated from p27Kip1+/+ and p27Kip1−/− MEFs with the Nuclear/Cytosol Fractionation kit. Western blotting assays were carried out to determine protein expression and phosphorylation as indicated. GAPDH and histone H3 were used as cytoplasmic and nuclear protein markers. (B) After pretreatment of cells with either control medium or 25 μM SP600125, cytosolic and nuclear proteins were isolated from p27Kip1−/− cells using the Nuclear/Cytosol Fractionation kit. The isolated proteins were subjected to Western blotting to determine protein expression and phosphorylation as indicated. GAPDH and histone H3 were used as cytoplasmic and nuclear protein markers. (C) Total cell lysates from p27Kip1−/− MEFs cells stably transfected with shRNA-JNK were subjected to Western blotting as indicated. α-Tubulin was used as a protein loading control. (D) Total cell lysates from p27Kip1−/− MEFs stably transfected with either DN-JNK or TAM67 were subjected to Western blotting as indicated. GAPDH was used as a protein loading control.