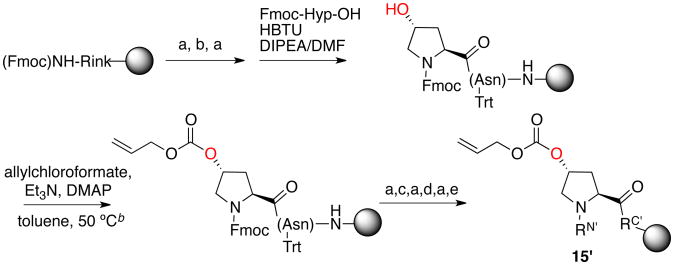
Scheme 3.
Proline editing alternative approach: synthesis of the peptide Ac-TYP(4R-O-Alloc)N-NH2(15) via Alloc protection and direct acylation. The Alloc group was stable to TFA cleavage/deprotection. a
a (a) 20% piperidine/DMF; (b) Fmoc-Asn(Trt)-OH, HBTU, DIPEA/DMF; (c) Fmoc-Tyr(OtBu)-OH, HBTU, DIPEA/DMF; (d) Fmoc-Thr(OtBu)-OH, HBTU, DIPEA/DMF; (e) 10% Ac2O/pyridine. RN′ = Ac-Thr(OtBu)-Tyr(OtBu)-, RC′ = -Asn(Trt)-NHRink-Resin. RN = Ac-Thr-Tyr-, RC = -Asn-NH2
b Reaction was performed in a glass vial manually.