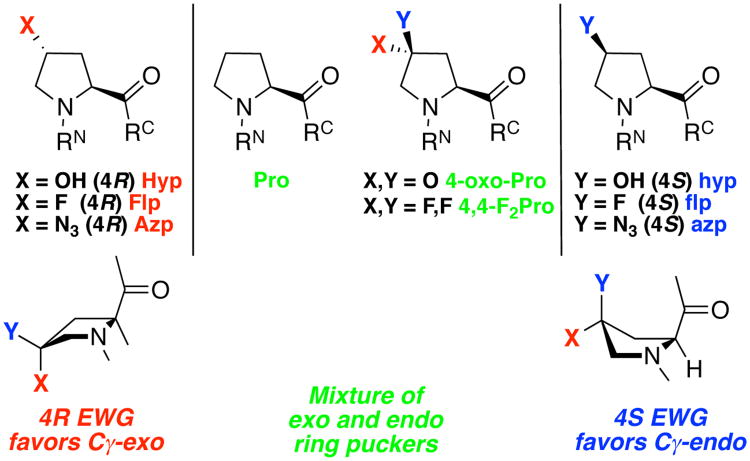
Figure 4.
Most-studied 4-substituted proline derivatives, three letter codes, and their conformational preferences. Red color and upper case 3-letter code indicates trans relative stereochemistry (4R substitution) of the 4-substituent and the carbonyl. Blue color and lower case 3-letter code indicates cis relative stereochemistry (4S) of the 4-substitutent and the carbonyl. Green color indicates 4,4-disubstituted prolines. EWG = electron-withdrawing group. Non-electron-withdrawing or sterically demanding substituents have opposite conformational preferences: 4R-substituted methylproline (X = CH3) and 4R-mercaptoproline (X = SH) favor Cγ-endo ring pucker, while 4S-substituted-methylproline and 4S-mercaptoproline favor Cγ-exoring pucker.7a, 11, 39