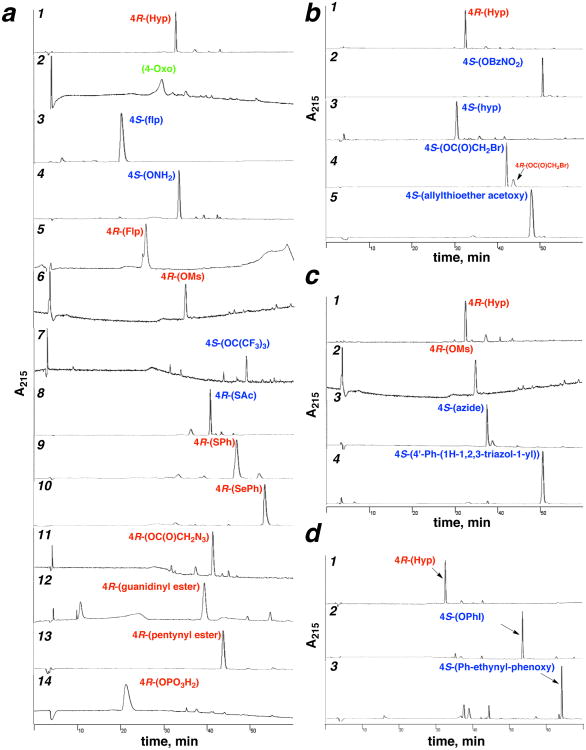
Figure 6.
Representative crude HPLC chromatograms of proline editing reactions on solid phase, including inversion of hydroxyproline stereochemistry, sulfonylation, SN2, Mitsunobu, acylation, oxidation, and solid phase organometallic reactions. HPLC chromatograms for the synthesis of all peptides are in the Supporting Information (Part 2). (a) Representative reaction products from Hyp (1), including oxidation (entry 2 (91)), DAST reaction (entries 3 (26) and 5 (27) (via hyp)), Mitsunobu reaction (entries 4 (52), 7 (56)), sulfonation (entry 6 (5)), 3-step sequences via Mitsunobu reaction on hyp (entries 8 (43), 9 (16), 10 (41)), acylation plus SN2 (entry 11 (83)), acylation (entries 12 (64), 13 (79)), and phosphorylation (entry 14 (97)). Entry 5 (Flp) includes flp as a minor impurity in the chromatogram due to incomplete Mitsunobu reaction to form the hyp starting material for the DAST reaction. (b) Synthesis of 4S-acetoxy allylthioether (90) from Hyp (1) via Mitsunobu inversion to the hyp nitrobenzoate (2), azidemediated deesterification to hyp (4), acetylation to the bromoacetate (75), and SN2 reaction with allyl mercaptan. (c) Synthesis of the 4S-azide-alkyne cycloaddition product (109) from Hyp (1) via mesylation (5), SN2 reaction with sodium azide (21), and copper-mediated Huisgen cycloaddition. (d) Synthesis of the 4S-Sonogashira product (107) from Hyp (1) via Mitsunobu reaction with 4-iodophenol (37) and Pd-mediated cross-coupling with phenylacetylene.