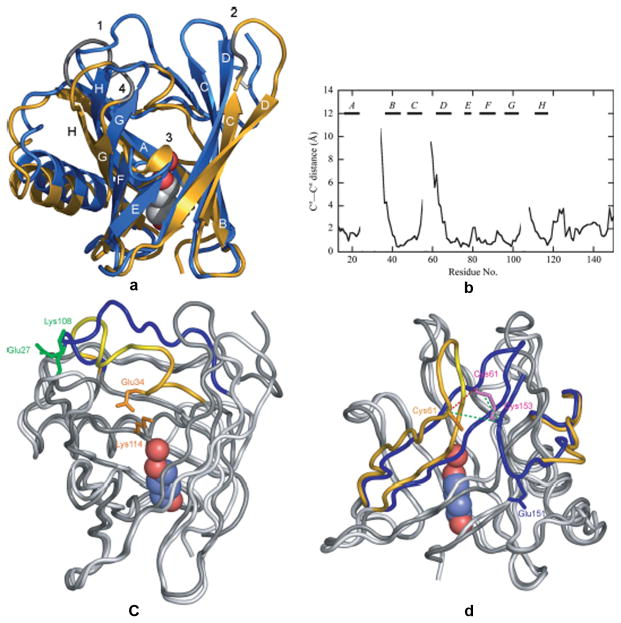
Figure 3.
Comparison of the TLC structures obtained from two crystal forms. A. Superposition of TLC crystallized in space group P21 (chain A, blue) and is space group C2 (gold with modeled residues in grey). The bound 1,4-butanediol and hydrogen-bonded water are represented as spheres and the conserved disulfide bond is shown in white. B. Pairwise Ca-atom distances between the two different TLC crystal structures after superposition of the 58 conserved Cα positions of the β-barrel. C. Electrostatic interactions that may trigger the two alternative conformations of loop 1. In the P21 structure (dark grey) Glu27 and Lys108 (green) make an electrostatic contact that fixes the “open” conformation of loop 1 (blue). The conformation of this loop in C2 structure (light grey) is stabilized by interaction between Glu34 and Lys114 (orange). D. Flexibility of the region around the disulfide bridges. The disulfide bond between Cys61 and Cys153 is well ordered in the P21 structure (dark grey; loop 2 and the C-terminal peptide segment (blue). Only Cys61 was resolved in the C2 structure (light grey; loop 2 and the C-terminal (gold). Reprinted from Breustedt, et al 2009.