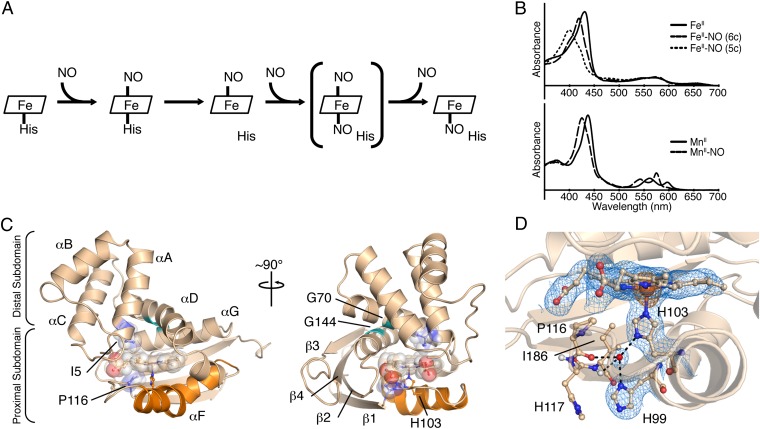
Fig. 1.
NO binding in H-NOX proteins and structural features of FeII-unligated So H-NOX. (A) Reaction scheme of NO binding to H-NOX proteins. The putative dinitrosyl intermediate is shown in brackets. (B) UV-visible absorption spectra for FeII (Upper) and MnII (Lower) nitrosyl complexes. The six-coordinate (6c) FeII nitrosyl intermediate spectrum (Upper, thick dashed line) was obtained via stopped-flow UV-visible spectroscopy (5c, five coordinate). (C) Cartoon representation of the FeII-unligated So H-NOX structure [molecule A of the asymmetric unit (ASU)]. Secondary structure elements and important structural features are highlighted. The signaling helix, αF, is displayed in orange; residues I5 and P116 and the heme are shown as transparent spheres; and conserved glycines G70 and G144 are shown in green. (D) Detailed view of the FeII-unligated H-NOX heme pocket showing a strongly hydrogen-bonded water molecule (shown as a red sphere) with residues in the proximal heme pocket. The 2mFo-DFc electron density (blue mesh, 1σ) and the anomalous difference density (orange mesh, 5σ) are displayed.