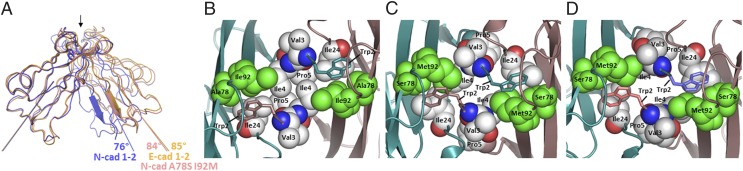
Fig. 3.
Structural importance of the Trp2 pocket lining residues 78 and 92. (A) Ribbon diagram representation of superposed type I classical cadherins’ strand-swapped dimers. EC1 domains of the crystal structure of E-cadherin (PDB ID code 2QVF) is shown in orange, N-cadherin wild-type (PDB ID code 2QVI) in blue, and the N-cadherin double-mutant A78S I92M in salmon. For all dimer structures, only the protomer in the foreground has been superposed. The A-strand of the other protomer highlights the difference of relative orientation of the two protomers within each swapped dimer. The long axis of each EC1 domain used to calculate dimer angles (Materials and Methods) is represented. Fig. S3 shows a similar superposition including other available type I cadherin swapped dimer crystal structures. (B–D) Detailed view of side-chain packing at and around the hydrophobic binding pocket of Trp2 for wild-type N-cadherin (B), wild-type E-cadherin (C), and N-cadherin A78S I92M double-mutant (D). In each panel, the swapped interface is viewed from the top, as indicated by the arrow at the top of A. The same crystal structures as in A have been used. The side-chains of residues lining the Trp2 pocket or part of the hydrophobic cluster around it are shown in Van der Waals sphere representation, except for the two Trp2 that are shown in stick representation for clarity. The only subtype-specific residues, 78 and 92, are shown in green. Note that the color coding is different from A, as in each dimer one protomer is represented in dark salmon and the other one in cyan.