Table 1. Optimization of Ni-Catalyzed Asymmetric Reductive Coupling.
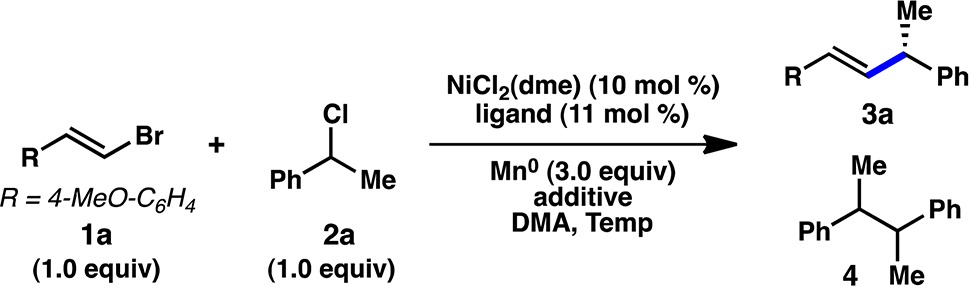
| entrya | ligand | additive | temp (°C) | yield 4 (%)b | yield 3a (%)b | ee 3a (%)c |
|---|---|---|---|---|---|---|
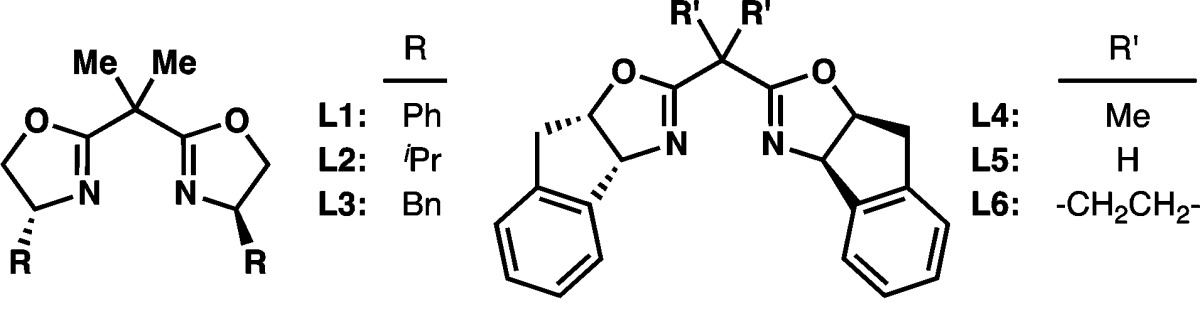
| 1 | L1 | – | 20 | 48 | 50 | 40 |
| 2 | L2 | – | 20 | 33 | 21 | 57 |
| 3 | L3 | – | 20 | 38 | 25 | 68 |
| 4 | L4 | – | 20 | 35 | 26 | 70 |
| 5 | L5 | – | 20 | 21 | 33 | 49 |
| 6 | L6 | – | 20 | 20 | 56 | 87 |
| 7 | L6 | TFA | 20 | 30 | 39 | 86 |
| 8 | L6 | TMSCl | 20 | 26 | 33 | 73 |
| 9 | L6 | NaId | 20 | 17 | 67 | 87 |
| 10 | L6 | TBAId | 20 | 13 | 64 | 91 |
| 11 | L6 | NaId | 0 | 8 | 93 | 93 |
| 12e | L6 | NaId | 0 | 8 | 69 | 89 |
| 13f | L6 | NaId | 0 | 0 | 0 | – |
| 14g | L6 | NaId | 0 | 0 | 0 | – |
| 15 | – | NaId | 0 | 0 | 0 | – |
Reactions conducted under N2 on 0.2 mmol scale for 6 h.
Determined by GC vs an internal standard.
Determined by SFC using a chiral stationary phase.
0.5 equiv.
Zn0 used instead of Mn0.
No Mn0.
No NiCl2(dme).