Table 2. Substrate Scope of Benzyl Chlorides.
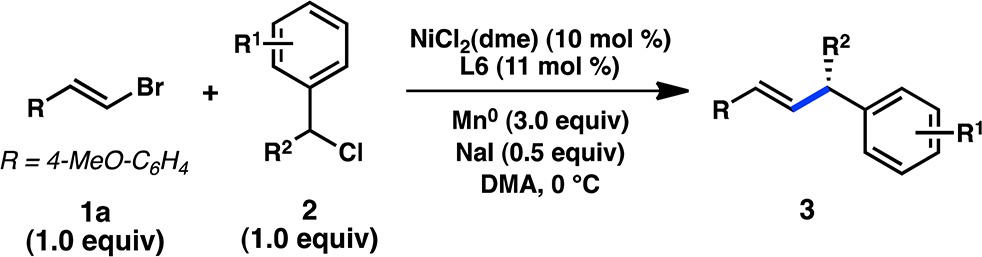
| entry | R1 | R2 | pdt | yield (%)a | ee (%)b |
|---|---|---|---|---|---|
| 1 | H | Me | 3a | 91 | 93 |
| 2 | 4-Me | Me | 3b | 82 | 94 |
| 3 | 3-Me | Me | 3c | 88 | 93 |
| 4c | 2-Me | Me | 3d | 44 | 85 |
| 5 | 4-OMe | Me | 3e | 64 | 93 |
| 6 | 4-F | Me | 3f | 81 | 89 |
| 7c | 4-Cl | Me | 3g | 75 | 88 |
| 8 | 4-Br | Me | 3h | 59 | 90 |
| 9c | 4-OCF3 | Me | 3i | 84 | 88 |
| 10 | H | Et | 3j | 80 | 97 |
| 11 | H | Bn | 3k | 82 | 93 |
| 12 | H | 4-pentenyl | 3l | 68 | 94 |
| 13 | H | 2-hydroxyethyl | 3m | 81 | 96 |
| 14c | H | 2-chloroethyl | 3n | 60 | 94 |
Isolated yield, reactions conducted under N2 on 0.2 mmol scale for 6 h.
Determined by SFC using a chiral stationary phase.
Run with 15 mol % NiCl2(dme) and 16 mol % L6.
