Figure 2.
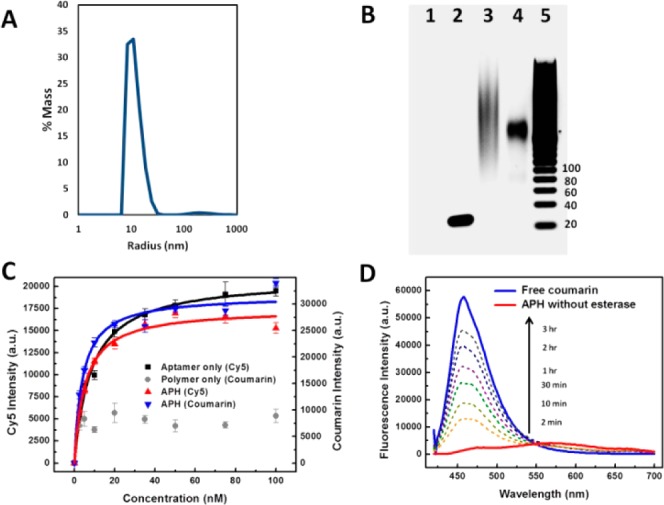
Characterizing functional integrity of the APH construct. (A) Dynamic light scattering measurements revealed a narrow radius distribution of ∼10 nm with negligible aggregation in phosphate-buffered saline buffer. (B) Gel electrophoresis shows that APH conjugation via (BimC4A)3 ligand-accelerated CuAAC greatly minimizes DNA damage. Lanes: 1, unconjugated polymer; 2, unconjugated aptamer (45 nt); 3, APH synthesized with (Bim)3; 4, APH synthesized with (BimC4A)3; 5, 20-bp ladder. (C) Affinity measurements from a bead-based nucleolin-binding assay show that aptamer target affinity is preserved within the APH construct. The unconjugated aptamer exhibits a Kd of 8.37 ± 0.75 nM (black), while APH molecules consisting of Cy5-labeled aptamer and coumarin-labeled polymer scaffold display similar Kds of 5.18 ± 0.72 and 4.01 ± 0.72 nM based on Cy5 (red) and coumarin (blue) intensities, respectively. In contrast, unconjugated polymer (gray) exhibits negligible nucleolin affinity. (D) Time-dependent fluorescence measurements confirm selective payload release. Untreated APHs emit a red-shifted, self-quenched signal (red), but esterase treatment shifts the peak fluorescence wavelength to that of free coumarin (blue), with a signal that increases over time as more coumarin is released (dashed lines).
